Beneficial Effects of Potentilla discolor Bunge Water Extract on Inflammatory Cytokines Release and Gut Microbiota in High-Fat Diet and Streptozotocin-Induced Type 2 Diabetic Mice
- PMID: 30897784
- PMCID: PMC6470731
- DOI: 10.3390/nu11030670
Beneficial Effects of Potentilla discolor Bunge Water Extract on Inflammatory Cytokines Release and Gut Microbiota in High-Fat Diet and Streptozotocin-Induced Type 2 Diabetic Mice
Abstract
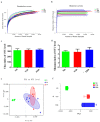
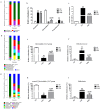
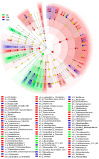
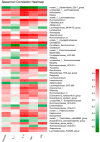
Potentilla discolor Bunge (PDB), a perennial herb, has been used as a traditional Chinese medicine in the therapy of many diseases. The aim of the current study was to investigate the effect of PDB water extract on systemic inflammation and gut microbiota in type 2 diabetic (T2D) mice induced by high-fat diet (HFD) and streptozotocin (STZ) injection. C57BL/6J mice were randomly divided into a normal diet (ND) group, T2D group, and PDB group (diabetic mice treated with PDB water extract at a dose of 400 mg/kg body weight). Results showed that PDB significantly decreased the levels of lipopolysaccharide (LPS) and pro-inflammatory cytokines in serum. Further investigation showed that PDB significantly reduced the ratio of Firmicutes/Bacteroidetes and the relative abundance of Proteobacteria in fecal samples of diabetic mice. In addition, PDB notably alleviated intestinal inflammation as evidenced by decreased expression of toll-like receptor 4 (TLR4), myeloid differentiation factor 88 (MyD88), nuclear factor-κB (NF-κB), and inflammatory cytokines. PDB also reversed the decreased expression of intestinal mucosal tight junction proteins including Claudin3, ZO-1, and Occludin. Meanwhile, the levels of fecal acetic acid and butyric acid and their specific receptors including G-protein-coupled receptor (GPR) 41 and 43 expression in the colon were also increased after PDB treatment. Our results indicated that PDB might serve as a potential functional ingredient against diabetes and related inflammation.
Keywords: Potentilla discolor Bunge water extract; diabetes; gut microbiota; inflammation; intestinal mucosal barrier function; short chain fatty acids.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Molecular mechanisms of Potentilla Discolor Bunge in regulating ferroptosis to alleviate DKD via the Nrf2 signaling pathway.J Ethnopharmacol. 2025 Jun 26;350:120035. doi: 10.1016/j.jep.2025.120035. Epub 2025 May 23. J Ethnopharmacol. 2025. PMID: 40414576
-
The anti-diabetic activities, gut microbiota composition, the anti-inflammatory effects of Scutellaria-coptis herb couple against insulin resistance-model of diabetes involving the toll-like receptor 4 signaling pathway.J Ethnopharmacol. 2019 Jun 12;237:202-214. doi: 10.1016/j.jep.2019.02.040. Epub 2019 Feb 23. J Ethnopharmacol. 2019. PMID: 30807814
-
Sodium butyrate attenuates high-fat diet-induced steatohepatitis in mice by improving gut microbiota and gastrointestinal barrier.World J Gastroenterol. 2017 Jan 7;23(1):60-75. doi: 10.3748/wjg.v23.i1.60. World J Gastroenterol. 2017. PMID: 28104981 Free PMC article.
-
Effects of potentilla discolor bunge extracts on oxidative stress and glycolipid metabolism in animal models of diabetes: a systematic review and meta-analysis.Front Pharmacol. 2023 Oct 2;14:1218757. doi: 10.3389/fphar.2023.1218757. eCollection 2023. Front Pharmacol. 2023. PMID: 37849729 Free PMC article.
-
Therapeutic potential of traditional Chinese medicine for the treatment of NAFLD: A promising drug Potentilla discolor Bunge.Acta Pharm Sin B. 2022 Sep;12(9):3529-3547. doi: 10.1016/j.apsb.2022.05.001. Epub 2022 May 11. Acta Pharm Sin B. 2022. PMID: 36176915 Free PMC article. Review.
Cited by
-
Dietary flaxseed oil rich in omega-3 suppresses severity of type 2 diabetes mellitus via anti-inflammation and modulating gut microbiota in rats.Lipids Health Dis. 2020 Feb 7;19(1):20. doi: 10.1186/s12944-019-1167-4. Lipids Health Dis. 2020. PMID: 32028957 Free PMC article.
-
Relationship between Microflora Changes and Mammary Lipid Metabolism in Dairy Cows with Mastitis.Animals (Basel). 2023 Aug 31;13(17):2773. doi: 10.3390/ani13172773. Animals (Basel). 2023. PMID: 37685037 Free PMC article.
-
Water-Soluble Cellulose Acetate Changes the Intestinal Microbiota in Mice with Non-Alcoholic Steatohepatitis.Nutrients. 2025 Jan 29;17(3):500. doi: 10.3390/nu17030500. Nutrients. 2025. PMID: 39940357 Free PMC article.
-
Peanut skin extract ameliorates the symptoms of type 2 diabetes mellitus in mice by alleviating inflammation and maintaining gut microbiota homeostasis.Aging (Albany NY). 2020 Jul 22;12(14):13991-14018. doi: 10.18632/aging.103521. Epub 2020 Jul 22. Aging (Albany NY). 2020. PMID: 32699185 Free PMC article.
-
Study of the Antimicrobial Activity of the Chinese Dong Ethnic Minority Medicine, Madeng'ai.Evid Based Complement Alternat Med. 2022 Aug 1;2022:3678240. doi: 10.1155/2022/3678240. eCollection 2022. Evid Based Complement Alternat Med. 2022. PMID: 35958918 Free PMC article.
References
-
- Larsen N., Vogensen F.K., van den Berg F.W.J., Nielsen D.S., Andreasen A.S., Pedersen B.K., Abu Al-Soud W., Sorensen S.J., Hansen L.H., Jakobsen M. Gut Microbiota in Human Adults with Type 2 Diabetes Differs from Non-Diabetic Adults. PLoS ONE. 2010;5:e9085. doi: 10.1371/journal.pone.0009085. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

