Single-Molecule Imaging and Computational Microscopy Approaches Clarify the Mechanism of the Dimerization and Membrane Interactions of Green Fluorescent Protein
- PMID: 30897814
- PMCID: PMC6471090
- DOI: 10.3390/ijms20061410
Single-Molecule Imaging and Computational Microscopy Approaches Clarify the Mechanism of the Dimerization and Membrane Interactions of Green Fluorescent Protein
Abstract
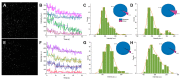
Green fluorescent protein (GFP) is widely used as a biomarker in living systems; however, GFP and its variants are prone to forming low-affinity dimers under physiological conditions. This undesirable tendency is exacerbated when fluorescent proteins (FP) are confined to membranes, fused to naturally-oligomeric proteins, or expressed at high levels in cells. Oligomerization of FPs introduces artifacts into the measurement of subunit stoichiometry, as well as interactions between proteins fused to FPs. Introduction of a single mutation, A206K, has been shown to disrupt hydrophobic interactions in the region responsible for GFP dimerization, thereby contributing to its monomerization. Nevertheless, a detailed understanding of how this single amino acid-dependent inhibition of dimerization in GFP occurs at the atomic level is still lacking. Single-molecule experiments combined with computational microscopy (atomistic molecular dynamics) revealed that the amino group of A206 contributes to GFP dimer formation via a multivalent electrostatic interaction. We further showed that myristoyl modification is an efficient mechanism to promote membrane attachment of GFP. Molecular dynamics-based site-directed mutagenesis has been used to identify the key functional residues in FPs. The data presented here have been utilized as a monomeric control in downstream single-molecule studies, facilitating more accurate stoichiometry quantification of functional protein complexes in living cells.
Keywords: N-myristoylation; molecular dynamics; single molecule; stoichiometry.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures




Similar articles
-
Assessing the tendency of fluorescent proteins to oligomerize under physiologic conditions.Traffic. 2012 May;13(5):643-9. doi: 10.1111/j.1600-0854.2012.01336.x. Epub 2012 Feb 20. Traffic. 2012. PMID: 22289035 Free PMC article.
-
A modified GFP facilitates counting membrane protein subunits by step-wise photobleaching in Arabidopsis.J Plant Physiol. 2017 Jun;213:129-133. doi: 10.1016/j.jplph.2017.03.009. Epub 2017 Mar 22. J Plant Physiol. 2017. PMID: 28380405
-
The Single-Molecule Approach to Membrane Protein Stoichiometry.Methods Mol Biol. 2016;1427:189-99. doi: 10.1007/978-1-4939-3615-1_11. Methods Mol Biol. 2016. PMID: 27259928
-
Fluorescent protein applications in plants.Methods Cell Biol. 2008;85:153-77. doi: 10.1016/S0091-679X(08)85008-X. Methods Cell Biol. 2008. PMID: 18155463 Review.
-
Advances in fluorescent protein technology.J Cell Sci. 2007 Dec 15;120(Pt 24):4247-60. doi: 10.1242/jcs.005801. J Cell Sci. 2007. PMID: 18057027 Review.
Cited by
-
Influence of nanobody binding on fluorescence emission, mobility, and organization of GFP-tagged proteins.iScience. 2020 Dec 4;24(1):101891. doi: 10.1016/j.isci.2020.101891. eCollection 2021 Jan 22. iScience. 2020. PMID: 33364580 Free PMC article.
-
Polyvinyl Chloride Nanoparticles Affect Cell Membrane Integrity by Disturbing the Properties of the Multicomponent Lipid Bilayer in Arabidopsis thaliana.Molecules. 2022 Sep 11;27(18):5906. doi: 10.3390/molecules27185906. Molecules. 2022. PMID: 36144641 Free PMC article.
-
Effects of small molecule-induced dimerization on the programmed death ligand 1 protein life cycle.Sci Rep. 2022 Dec 9;12(1):21286. doi: 10.1038/s41598-022-25417-6. Sci Rep. 2022. PMID: 36494467 Free PMC article.
-
Electromechanical Photophysics of GFP Packed Inside Viral Protein Cages Probed by Force-Fluorescence Hybrid Single-Molecule Microscopy.Small. 2022 Jul;18(28):e2200059. doi: 10.1002/smll.202200059. Epub 2022 Jun 19. Small. 2022. PMID: 35718881 Free PMC article.
-
The nanoscale organization of Nipah virus matrix protein revealed by super-resolution microscopy.Biophys J. 2022 Jun 21;121(12):2290-2296. doi: 10.1016/j.bpj.2022.05.026. Epub 2022 May 25. Biophys J. 2022. PMID: 35614854 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

