Preparation of Sesquiterpene Lactone Derivatives: Cytotoxic Activity and Selectivity of Action
- PMID: 30897836
- PMCID: PMC6471591
- DOI: 10.3390/molecules24061113
Preparation of Sesquiterpene Lactone Derivatives: Cytotoxic Activity and Selectivity of Action
Abstract
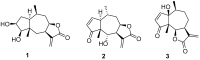
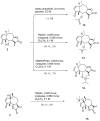
Cancer is one of the most important causes of death worldwide. Solid tumors represent the great majority of cancers (>90%) and the chemotherapeutic agents used for their treatment are still characterized by variable efficacy and toxicity. Sesquiterpene lactones are a group of naturally occurring compounds that have displayed a diverse range of biological activities including cytotoxic activity. A series of oxygenated and oxy-nitrogenated derivatives (4⁻15) from the sesquiterpene lactones cumanin (1), helenalin (2), and hymenin (3) were synthesized. The silylated derivatives of helenalin, compounds 13 and 14, were found to be the most active against tumor cell lines, with GI50 values ranging from 0.15 to 0.59 μM. The ditriazolyl cumanin derivative (11) proved to be more active and selective than cumanin in the tested breast, cervix, lung, and colon tumor cell lines. This compound was the least toxic against splenocytes (CC50 = 524.1 µM) and exhibited the greatest selectivity on tumor cell lines. This compound showed a GI50 of 2.3 µM and a SI of 227.9 on WiDr human colon tumor cell lines. Thus, compound 11 can be considered for further studies and is a candidate for the development of new antitumor agents.
Keywords: Asteraceae; antiproliferative activity; cumanin; helenalin; hymenin; sesquiterpene lactones.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Cytotoxic sesquiterpene lactones mediate their death-inducing effect in leukemia T cells by triggering apoptosis.Planta Med. 2001 Aug;67(6):557-9. doi: 10.1055/s-2001-16478. Planta Med. 2001. PMID: 11509981
-
Sesquiterpene lactones from the extracts of two Balkan endemic Laserpitium species and their cytotoxic activity.Phytochemistry. 2013 Mar;87:102-11. doi: 10.1016/j.phytochem.2012.11.011. Epub 2012 Dec 12. Phytochemistry. 2013. PMID: 23246199
-
Cajamolides A-N: Cytotoxic and anti-inflammatory sesquiterpene lactones from Calea jamaicensis.Bioorg Chem. 2021 Nov;116:105351. doi: 10.1016/j.bioorg.2021.105351. Epub 2021 Sep 11. Bioorg Chem. 2021. PMID: 34583199
-
Antitumor activity and mechanism of costunolide and dehydrocostus lactone: Two natural sesquiterpene lactones from the Asteraceae family.Biomed Pharmacother. 2020 May;125:109955. doi: 10.1016/j.biopha.2020.109955. Epub 2020 Jan 31. Biomed Pharmacother. 2020. PMID: 32014691 Review.
-
Development of Anticancer Agents from Plant-Derived Sesquiterpene Lactones.Curr Med Chem. 2016;23(23):2397-420. doi: 10.2174/0929867323666160510123255. Curr Med Chem. 2016. PMID: 27160533 Free PMC article. Review.
Cited by
-
A comprehensive phytochemical and pharmacological review on sesquiterpenes from the genus Ambrosia.Heliyon. 2022 Jul 9;8(7):e09884. doi: 10.1016/j.heliyon.2022.e09884. eCollection 2022 Jul. Heliyon. 2022. PMID: 35865986 Free PMC article. Review.
-
Sesquiterpene Lactones and Diterpenes: Promising Therapeutic Candidates for Infectious Diseases, Neoplasms and Other Chronic Disorders.Molecules. 2021 Feb 26;26(5):1251. doi: 10.3390/molecules26051251. Molecules. 2021. PMID: 33652593 Free PMC article.
-
Phyto-Sesquiterpene Lactones Prevent the Development of Multidrug Resistance in TNBC via ABC Transporters Inhibition and STAT3/MYC Signaling.Cancers (Basel). 2025 Apr 14;17(8):1321. doi: 10.3390/cancers17081321. Cancers (Basel). 2025. PMID: 40282497 Free PMC article.
-
Oxonitrogenated Derivatives of Eremophilans and Eudesmans: Antiproliferative and Anti-Trypanosoma cruzi Activity.Molecules. 2022 May 10;27(10):3067. doi: 10.3390/molecules27103067. Molecules. 2022. PMID: 35630539 Free PMC article.
-
In Vitro, In Vivo, and In Silico Studies of Cumanin Diacetate as a Potential Drug against Trypanosoma cruzi Infection.ACS Omega. 2021 Dec 20;7(1):968-978. doi: 10.1021/acsomega.1c05560. eCollection 2022 Jan 11. ACS Omega. 2021. PMID: 35036760 Free PMC article.
References
-
- Lahlou M. The success of natural products in drug discovery. Pharmacol. Pharm. 2013;4:17–31. doi: 10.4236/pp.2013.43A003. - DOI
-
- Sülsen V.P., Frank F.M., Cazorla S.I., Anesini C.A., Malchiodi E.L., Freixa B., Martino V.S. Trypanocidal and leishmanicidal activities of sesquiterpene lactones from Ambrosia tenuifolia Sprengel (Asteraceae) Antimicrob. Agent. Chemother. 2008;52:2415–2419. doi: 10.1128/AAC.01630-07. - DOI - PMC - PubMed
-
- Sülsen V.P., Cazorla S.I., Frank F.M., Laurella L.C., Muschietti L.V., Catalan C.A., Malchiodi E.L. Natural terpenoids from Ambrosia species are active in vitro and in vivo against human pathogenic trypanosomatids. PLoS Negl. Trop. Dis. 2013;7:e2494. doi: 10.1371/journal.pntd.0002494. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
- PIP-090/Consejo Nacional de Investigaciones Científicas y Técnicas
- PROICO 2-2516/Universidad Nacional de San Luis
- PIP 11220150100158CO/Consejo Nacional de Investigaciones Científicas y Técnicas
- PICT 2015-3531/Agencia Nacional de Promoción Científica y Tecnológica
- UBACYT 20020130200270/Universidad de Buenos Aires
LinkOut - more resources
Full Text Sources

