Cysteine Cathepsins and their Extracellular Roles: Shaping the Microenvironment
- PMID: 30897858
- PMCID: PMC6468544
- DOI: 10.3390/cells8030264
Cysteine Cathepsins and their Extracellular Roles: Shaping the Microenvironment
Abstract
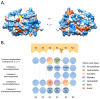
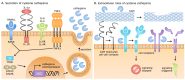
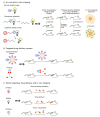
: For a long time, cysteine cathepsins were considered primarily as proteases crucial for nonspecific bulk proteolysis in the endolysosomal system. However, this view has dramatically changed, and cathepsins are now considered key players in many important physiological processes, including in diseases like cancer, rheumatoid arthritis, and various inflammatory diseases. Cathepsins are emerging as important players in the extracellular space, and the paradigm is shifting from the degrading enzymes to the enzymes that can also specifically modify extracellular proteins. In pathological conditions, the activity of cathepsins is often dysregulated, resulting in their overexpression and secretion into the extracellular space. This is typically observed in cancer and inflammation, and cathepsins are therefore considered valuable diagnostic and therapeutic targets. In particular, the investigation of limited proteolysis by cathepsins in the extracellular space is opening numerous possibilities for future break-through discoveries. In this review, we highlight the most important findings that establish cysteine cathepsins as important players in the extracellular space and discuss their roles that reach beyond processing and degradation of extracellular matrix (ECM) components. In addition, we discuss the recent developments in cathepsin research and the new possibilities that are opening in translational medicine.
Keywords: cancer; cathepsin; extracellular matrix; inflammation associated disease; osteoporosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Vizovisek M., Vidmar R., Van Quickelberghe E., Impens F., Andjelkovic U., Sobotic B., Stoka V., Gevaert K., Turk B., Fonovic M. Fast profiling of protease specificity reveals similar substrate specificities for cathepsins K, L and S. Proteomics. 2015;15:2479–2490. doi: 10.1002/pmic.201400460. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

