Adipose-derived stem cells promote survival, growth, and maturation of early-stage murine follicles
- PMID: 30898159
- PMCID: PMC6427888
- DOI: 10.1186/s13287-019-1199-8
Adipose-derived stem cells promote survival, growth, and maturation of early-stage murine follicles
Abstract
Background: Premature ovarian insufficiency is a common complication of anticancer treatments in young women and girls. The ovary is a complex, highly regulated reproductive organ, whose proper function is contingent upon the bidirectional endocrine, paracrine, and autocrine signaling. These factors facilitate the development of the follicles, the functional units of the ovary, to progress from the gonadotropin-independent, paracrine-controlled early stage to the gonadotropin-dependent, endocrine-controlled later stage. We hypothesized that the low survival rate of individually cultured early-stage follicles could be improved with co-culture of adipose-derived stem cells (ADSCs) that secrete survival- and growth-promoting factors.
Materials and methods: Ovarian follicles ranging from 85 to 115 μm in diameter, from 10- to 12-day-old B6CBAF1 mice were mechanically isolated and co-encapsulated with ADSCs within alginate-based 3D culture system. The follicles were cultured for 14 days, imaged using light microscopy every 2 days, and matured at the end. Follicle media were changed every 2 days and collected for hormone measurements. Follicle diameter, morphology, number of transzonal projections, and survival and maturation rates were recorded. Statistical analyses using one- and two-way ANOVA were performed to compare hormone levels, survival of the follicles and ADSCs, oocyte maturation rates, and follicle growth.
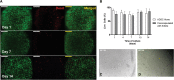
Results: The co-encapsulation of the follicles with ADSCs increased follicle survival, ranging from 42.4% for the 86-95 μm to 86.2% for the 106-115-μm follicle size group. Co-culture also improved the follicle growth, the rate of antrum formation and oocyte maturation compared to the follicles cultured alone. The levels of androstenedione, estradiol, and progesterone of co-encapsulated follicles increased progressively with time in culture.
Conclusions: To our knowledge, this is the first report of an in vitro system utilizing mouse adipose-derived stem cells to support the development of the mouse follicles. Our findings suggest that co-encapsulation of ADSCs with early-stage follicles supports follicular development, through secretion of cytokines that promote follicular survival, antrum formation, and meiotic competence. The unique 3D culture system that supports the survival of both cell types has translational implications, as ADSCs could be used as an autologous source for in vitro maturation of early-stage human follicles.
Keywords: 3D culture; Adipose-derived stem cells; Ovarian follicle.
Conflict of interest statement
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors have consented for publication.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures





Similar articles
-
Fibrin promotes development and function of macaque primary follicles during encapsulated three-dimensional culture.Hum Reprod. 2013 Aug;28(8):2187-200. doi: 10.1093/humrep/det093. Epub 2013 Apr 21. Hum Reprod. 2013. PMID: 23608357 Free PMC article.
-
Matrix-free 3D culture supports human follicular development from the unilaminar to the antral stage in vitro yielding morphologically normal metaphase II oocytes.Hum Reprod. 2021 Apr 20;36(5):1326-1338. doi: 10.1093/humrep/deab003. Hum Reprod. 2021. PMID: 33681988 Free PMC article.
-
Direct actions of androgens on the survival, growth and secretion of steroids and anti-Müllerian hormone by individual macaque follicles during three-dimensional culture.Hum Reprod. 2015 Mar;30(3):664-74. doi: 10.1093/humrep/deu335. Epub 2015 Jan 6. Hum Reprod. 2015. PMID: 25567619 Free PMC article.
-
Microfluidic Encapsulation of Ovarian Follicles for 3D Culture.Ann Biomed Eng. 2017 Jul;45(7):1676-1684. doi: 10.1007/s10439-017-1823-7. Epub 2017 Mar 20. Ann Biomed Eng. 2017. PMID: 28321583 Free PMC article. Review.
-
Immunoregulation of follicular renewal, selection, POF, and menopause in vivo, vs. neo-oogenesis in vitro, POF and ovarian infertility treatment, and a clinical trial.Reprod Biol Endocrinol. 2012 Nov 23;10:97. doi: 10.1186/1477-7827-10-97. Reprod Biol Endocrinol. 2012. PMID: 23176151 Free PMC article. Review.
Cited by
-
Drug-free in vitro activation combined with ADSCs-derived exosomes restores ovarian function of rats with premature ovarian insufficiency.J Ovarian Res. 2024 Jul 31;17(1):158. doi: 10.1186/s13048-024-01475-4. J Ovarian Res. 2024. PMID: 39085868 Free PMC article.
-
IL-37 Gene Modification Enhances the Protective Effects of Mesenchymal Stromal Cells on Intestinal Ischemia Reperfusion Injury.Stem Cells Int. 2020 Aug 7;2020:8883636. doi: 10.1155/2020/8883636. eCollection 2020. Stem Cells Int. 2020. PMID: 32849879 Free PMC article.
-
Ovarian Stromal Cell-Conditioned Media, but Not Co-Culture, Improves Survival in Feline Follicles.Animals (Basel). 2025 May 24;15(11):1539. doi: 10.3390/ani15111539. Animals (Basel). 2025. PMID: 40509005 Free PMC article.
-
Modulating adipose-derived stromal cells' secretomes by culture conditions: effects on angiogenesis, collagen deposition, and immunomodulation.Biosci Rep. 2025 Apr 23;45(5):325-42. doi: 10.1042/BSR20241389. Online ahead of print. Biosci Rep. 2025. PMID: 40267340 Free PMC article.
-
HucMSC-EVs Facilitate In Vitro Development of Maternally Aged Preantral Follicles and Oocytes.Stem Cell Rev Rep. 2023 Jul;19(5):1427-1448. doi: 10.1007/s12015-022-10495-w. Epub 2023 Mar 2. Stem Cell Rev Rep. 2023. PMID: 36862330 Free PMC article.
References
-
- Siegel R, et al. Cancer statistics, 2014. CA Cancer J Clin. 2014;64(1):9–29. - PubMed
-
- David A, Green LJ, Shikanov A. Fertility preservation in 2016: where are we? Semin Reprod Med. 2017;35(2):160-66. - PubMed
-
- Wallace WHB, Kelsey TW, Anderson RA. Fertility preservation in pre-pubertal girls with cancer: the role of ovarian tissue cryopreservation. Fertil Steril. 2016;105(1):6–12. - PubMed
-
- Woodruff TK. Reproductive endocrinology: fertility in female survivors of childhood cancer. Nat Rev Endocrinol. 2013;9(10):571. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

