Plasma copeptin levels predict disease progression and tolvaptan efficacy in autosomal dominant polycystic kidney disease
- PMID: 30898339
- PMCID: PMC6640141
- DOI: 10.1016/j.kint.2018.11.044
Plasma copeptin levels predict disease progression and tolvaptan efficacy in autosomal dominant polycystic kidney disease
Abstract
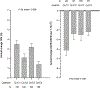
In the TEMPO 3:4 Trial, treatment with tolvaptan, a vasopressin V2 receptor antagonist, slowed the increase in total kidney volume and decline in estimated glomerular filtration rate (eGFR) in autosomal dominant polycystic kidney disease (ADPKD). We investigated whether plasma copeptin levels, a marker of plasma vasopressin, are associated with disease progression, and whether pre-treatment copeptin and treatment-induced change in copeptin are associated with tolvaptan treatment efficacy. This post hoc analysis included 1,280 TEMPO 3:4 participants (aged 18-50 years, estimated creatinine clearance ≥60 ml/min and total kidney volume ≥750 mL) who had plasma samples available at baseline for measurement of copeptin using an automated immunofluorescence assay. In placebo-treated subjects, baseline copeptin predicted kidney growth and eGFR decline over 3 years. These associations were independent of sex, age, and baseline eGFR, but were no longer statistically significant after additional adjustment for baseline total kidney volume. In tolvaptan-treated subjects, copeptin increased from baseline to week 3 (6.3 pmol/L versus 21.9 pmol/L, respectively). In tolvaptan-treated subjects with higher baseline copeptin levels, a larger treatment effect was noted with respect to kidney growth rate and eGFR decline. Tolvaptan-treated subjects with a larger percentage increase in copeptin from baseline to week 3 had a better disease outcome, with less kidney growth and eGFR decline after three years. Copeptin holds promise as a biomarker to predict outcome and tolvaptan treatment efficacy in ADPKD.
Keywords: ADPKD; AVP; copeptin; polycystic kidney disease; tolvaptan; vasopressin.
Copyright © 2019 International Society of Nephrology. All rights reserved.
Figures



Comment in
-
What can copeptin tell us in patients with autosomal dominant polycystic disease?Kidney Int. 2019 Jul;96(1):19-22. doi: 10.1016/j.kint.2019.02.037. Kidney Int. 2019. PMID: 31229028
Similar articles
-
Short-term Effects of Tolvaptan in Individuals With Autosomal Dominant Polycystic Kidney Disease at Various Levels of Kidney Function.Am J Kidney Dis. 2015 Jun;65(6):833-41. doi: 10.1053/j.ajkd.2014.11.010. Epub 2015 Jan 15. Am J Kidney Dis. 2015. PMID: 25600953
-
Can we further enrich autosomal dominant polycystic kidney disease clinical trials for rapidly progressive patients? Application of the PROPKD score in the TEMPO trial.Nephrol Dial Transplant. 2018 Apr 1;33(4):645-652. doi: 10.1093/ndt/gfx188. Nephrol Dial Transplant. 2018. PMID: 28992127 Free PMC article. Clinical Trial.
-
Change in kidney volume growth rate and renal outcomes of tolvaptan treatment in autosomal dominant polycystic kidney disease: post-hoc analysis of TEMPO 3:4 trial.Clin Exp Nephrol. 2025 May;29(5):638-649. doi: 10.1007/s10157-024-02589-1. Epub 2025 Jan 2. Clin Exp Nephrol. 2025. PMID: 39747793 Free PMC article. Clinical Trial.
-
An update on tolvaptan for autosomal dominant polycystic kidney disease.Drugs Today (Barc). 2018 Sep;54(9):519-533. doi: 10.1358/dot.2018.54.9.2776624. Drugs Today (Barc). 2018. PMID: 30303493 Review.
-
Tolvaptan: A Review in Autosomal Dominant Polycystic Kidney Disease.Drugs. 2015 Oct;75(15):1797-806. doi: 10.1007/s40265-015-0475-x. Drugs. 2015. PMID: 26407729 Review.
Cited by
-
Estimation of Changes in Kidney Volume Growth Rate in ADPKD.Kidney Int Rep. 2020 Jun 20;5(9):1459-1471. doi: 10.1016/j.ekir.2020.06.011. eCollection 2020 Sep. Kidney Int Rep. 2020. PMID: 32954070 Free PMC article.
-
DKK3 as a potential novel biomarker in patients with autosomal polycystic kidney disease.Clin Kidney J. 2023 Oct 13;17(1):sfad262. doi: 10.1093/ckj/sfad262. eCollection 2024 Jan. Clin Kidney J. 2023. PMID: 38186869 Free PMC article.
-
Prospective Study on Individualized Dose Adjustment of Tolvaptan Based on Urinary Osmolality in Patients With ADPKD.Kidney Int Rep. 2024 Jan 12;9(4):1031-1039. doi: 10.1016/j.ekir.2024.01.020. eCollection 2024 Apr. Kidney Int Rep. 2024. PMID: 38765583 Free PMC article.
-
Interventions for preventing the progression of autosomal dominant polycystic kidney disease.Cochrane Database Syst Rev. 2024 Oct 2;10(10):CD010294. doi: 10.1002/14651858.CD010294.pub3. Cochrane Database Syst Rev. 2024. PMID: 39356039
-
A Systematic Review of Reported Outcomes in ADPKD Studies.Kidney Int Rep. 2022 Jul 5;7(9):1964-1979. doi: 10.1016/j.ekir.2022.06.012. eCollection 2022 Sep. Kidney Int Rep. 2022. PMID: 36090492 Free PMC article.
References
-
- Gabow PA, Johnson AM, Kaehny WD, et al. Factors affecting the progression of renal disease in autosomal-dominant polycystic kidney disease. Kidney Int. 1992;41:1311–1319. - PubMed
-
- Gansevoort RT, Arici M, Benzing T, et al. Recommendations for the use of tolvaptan in autosomal dominant polycystic kidney disease: a position statement on behalf of the ERA-EDTA working groups on inherited kidney disorders and european renal best practice. Nephrol Dial Transplant. 2016;31:337–348. - PMC - PubMed
-
- Devuyst O, Torres VE. Osmoregulation, vasopressin, and cAMP signaling in autosomal dominant polycystic kidney disease. Curr Opin Nephrol Hypertens. 2013;22:459–470. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

