Identification of mammalian glycoproteins with type-I LacdiNAc structures synthesized by the glycosyltransferase B3GALNT2
- PMID: 30898876
- PMCID: PMC6509504
- DOI: 10.1074/jbc.RA118.006892
Identification of mammalian glycoproteins with type-I LacdiNAc structures synthesized by the glycosyltransferase B3GALNT2
Abstract
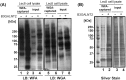
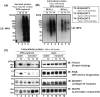
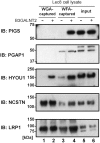
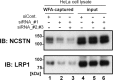
The type-I LacdiNAc (LDN; GalNAcβ1-3GlcNAc) has rarely been observed in mammalian cells except in the O-glycan of α-dystroglycan, in contrast to type-II LDN structures (GalNAcβ1-4GlcNAc) in N- and O-glycans that are present in many mammalian glycoproteins, such as pituitary and hypothalamic hormones. Although a β1,3-N-acetylgalactosaminyltransferase 2 (B3GALNT2; type-I LDN synthase) has been cloned, the function of type-I LDN in mammalian cells is still unclear, as its carrier protein(s) has not been identified. In this study, using HeLa cells, we demonstrate that inhibition of Golgi-resident glycosyltransferase increases the abundance of B3GALNT2-synthesized type-I LDN structures, recognized by Wisteria floribunda agglutinin (WFA). Using isotope-coded glycosylation site-specific tagging (IGOT)-LC/MS analysis of Lec8 Chinese hamster cells lacking galactosylation and of cells transfected with the B3GALNT2 gene, we identified the glycoproteins that carry B3GALNT2-generated type-I LDN in their N-glycans. Our results further revealed that LDN presence on low-density lipoprotein receptor-related protein 1 and nicastrin depends on B3GALNT2, indicating the occurrence of type-I LDN in vivo in mammalian cells. Our analysis also uncovered that most of the identified glycoproteins localize to intracellular organelles, particularly to the endoplasmic reticulum. Whereas B4GALNT3 and B4GALNT4 synthesized LDN on extracellular glycoproteins, B3GALNT2 primarily transferred LDN to intracellular glycoproteins, thereby clearly delineating proteins that carry type-I or type-II LDNs. Taken together, our results indicate the presence of mammalian glycoproteins carrying type-I LDN on N-glycans and suggest that type-I and type-II LDNs have different roles in vivo.
Keywords: B3GALNT2; Golgi; LC/MS analysis; N-linked glycosylation; WFA; Wisteria floribunda agglutinin (WFA); carbohydrate structure; endoplasmic reticulum; endoplasmic reticulum (ER); glycoprotein; glycosyltransferase; intracellular glycoproteins; type-I LacdiNAc.
© 2019 Nakane et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures




Similar articles
-
Novel poly-GalNAcbeta1-4GlcNAc (LacdiNAc) and fucosylated poly-LacdiNAc N-glycans from mammalian cells expressing beta1,4-N-acetylgalactosaminyltransferase and alpha1,3-fucosyltransferase.J Biol Chem. 2005 Apr 1;280(13):12810-9. doi: 10.1074/jbc.M414273200. Epub 2005 Jan 14. J Biol Chem. 2005. PMID: 15653684
-
Differential expression of LacdiNAc sequences (GalNAc beta 1-4GlcNAc-R) in glycoproteins synthesized by Chinese hamster ovary and human 293 cells.Glycobiology. 1997 Mar;7(2):183-94. doi: 10.1093/glycob/7.2.183. Glycobiology. 1997. PMID: 9134425
-
LacdiNAc synthase B4GALNT3 has a unique PA14 domain and suppresses N-glycan capping.J Biol Chem. 2024 Jul;300(7):107450. doi: 10.1016/j.jbc.2024.107450. Epub 2024 Jun 4. J Biol Chem. 2024. PMID: 38844136 Free PMC article.
-
Biosynthesis and Biological Significances of LacdiNAc Group on N- and O-Glycans in Human Cancer Cells.Biomolecules. 2022 Jan 24;12(2):195. doi: 10.3390/biom12020195. Biomolecules. 2022. PMID: 35204696 Free PMC article. Review.
-
Wisteria floribunda agglutinin positive glycobiomarkers: a unique lectin as a serum biomarker probe in various diseases.Expert Rev Proteomics. 2018 Feb;15(2):183-190. doi: 10.1080/14789450.2018.1419066. Epub 2017 Dec 21. Expert Rev Proteomics. 2018. PMID: 29265940 Review.
Cited by
-
Tamoxifen-resistant breast cancer cells exhibit reactivity with Wisteria floribunda agglutinin.PLoS One. 2022 Aug 25;17(8):e0273513. doi: 10.1371/journal.pone.0273513. eCollection 2022. PLoS One. 2022. PMID: 36006984 Free PMC article.
-
A phylogenetic view and functional annotation of the animal β1,3-glycosyltransferases of the GT31 CAZy family.Glycobiology. 2021 Apr 1;31(3):243-259. doi: 10.1093/glycob/cwaa086. Glycobiology. 2021. PMID: 32886776 Free PMC article.
-
Glycan Complexity and Heterogeneity of Glycoproteins in Somatic Extracts and Secretome of the Infective Stage of the Helminth Fasciola hepatica.Mol Cell Proteomics. 2023 Dec;22(12):100684. doi: 10.1016/j.mcpro.2023.100684. Epub 2023 Nov 21. Mol Cell Proteomics. 2023. PMID: 37993102 Free PMC article.
-
Low Collision Energy Fragmentation in Structure-Specific Glycoproteomics Analysis.Anal Chem. 2020 Jun 16;92(12):8262-8267. doi: 10.1021/acs.analchem.0c00519. Epub 2020 Jun 4. Anal Chem. 2020. PMID: 32441515 Free PMC article.
-
Differentially methylated regions interrogated for metastable epialleles associate with offspring adiposity.Epigenomics. 2024;16(18):1215-1230. doi: 10.1080/17501911.2024.2359365. Epub 2024 Sep 12. Epigenomics. 2024. PMID: 39263873 Free PMC article.
References
-
- Green E. D., van Halbeek H., Boime I., and Baenziger J. U. (1985) Structural elucidation of the disulfated oligosaccharide from bovine lutropin. J. Biol. Chem. 260, 15623–15630 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

