Autologous grafting of cryopreserved prepubertal rhesus testis produces sperm and offspring
- PMID: 30898927
- PMCID: PMC6598202
- DOI: 10.1126/science.aav2914
Autologous grafting of cryopreserved prepubertal rhesus testis produces sperm and offspring
Erratum in
-
Erratum for the Research Article "Autologous grafting of cryopreserved prepubertal rhesus testis produces sperm and offspring" by A. P. Fayomi, K. Peters, M. Sukhwani, H. Valli-Pulaski, G. Shetty, M. L. Meistrich, L. Houser, N. Robertson, V. Roberts, C. Ramsey, C. Hanna, J. D. Hennebold, I. Dobrinski, K. E. Orwig.Science. 2019 Apr 5;364(6435):eaax4999. doi: 10.1126/science.aax4999. Science. 2019. PMID: 30948525 No abstract available.
Abstract
Testicular tissue cryopreservation is an experimental method to preserve the fertility of prepubertal patients before they initiate gonadotoxic therapies for cancer or other conditions. Here we provide the proof of principle that cryopreserved prepubertal testicular tissues can be autologously grafted under the back skin or scrotal skin of castrated pubertal rhesus macaques and matured to produce functional sperm. During the 8- to 12-month observation period, grafts grew and produced testosterone. Complete spermatogenesis was confirmed in all grafts at the time of recovery. Graft-derived sperm were competent to fertilize rhesus oocytes, leading to preimplantation embryo development, pregnancy, and the birth of a healthy female baby. Pending the demonstration that similar results are obtained in noncastrated recipients, testicular tissue grafting may be applied in the clinic.
Copyright © 2019 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Figures



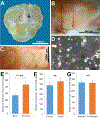
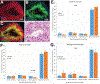

Comment in
-
Stem cell-based options to preserve male fertility.Science. 2019 Mar 22;363(6433):1283-1284. doi: 10.1126/science.aaw6927. Science. 2019. PMID: 30898920 No abstract available.
-
Grady - the miracle monkey born from cryopreserved prepubertal testis graft.Nat Rev Urol. 2019 Jun;16(6):329. doi: 10.1038/s41585-019-0182-6. Nat Rev Urol. 2019. PMID: 30980068 No abstract available.
-
Live Offspring after Testis Tissue Transplantation.N Engl J Med. 2019 Oct 10;381(15):1477-1479. doi: 10.1056/NEJMcibr1906768. N Engl J Med. 2019. PMID: 31597025 No abstract available.
References
-
- Levine J, Canada A, Stern CJ, Fertility preservation in adolescents and young adults with cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 28, 4831–4841 (2010). - PubMed
-
- Wallace WH, Anderson RA, Irvine DS, Fertility preservation for young patients with cancer: who is at risk and what can be offered? The lancet oncology 6, 209–218 (2005). - PubMed
-
- Goossens E, Van Saen D, Tournaye H, Spermatogonial stem cell preservation and transplantation: from research to clinic. Human Reproduction 28, 897–907 (2013). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

