Capturing site-specific heterogeneity with large-scale N-glycoproteome analysis
- PMID: 30899004
- PMCID: PMC6428843
- DOI: 10.1038/s41467-019-09222-w
Capturing site-specific heterogeneity with large-scale N-glycoproteome analysis
Abstract
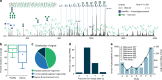
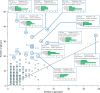
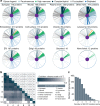
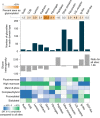
Protein glycosylation is a highly important, yet poorly understood protein post-translational modification. Thousands of possible glycan structures and compositions create potential for tremendous site heterogeneity. A lack of suitable analytical methods for large-scale analyses of intact glycopeptides has limited our abilities both to address the degree of heterogeneity across the glycoproteome and to understand how this contributes biologically to complex systems. Here we show that N-glycoproteome site-specific microheterogeneity can be captured via large-scale glycopeptide profiling methods enabled by activated ion electron transfer dissociation (AI-ETD), ultimately characterizing 1,545 N-glycosites (>5,600 unique N-glycopeptides) from mouse brain tissue. Our data reveal that N-glycosylation profiles can differ between subcellular regions and structural domains and that N-glycosite heterogeneity manifests in several different forms, including dramatic differences in glycosites on the same protein. Moreover, we use this large-scale glycoproteomic dataset to develop several visualizations that will prove useful for analyzing intact glycopeptides in future studies.
Conflict of interest statement
J.J.C. is an inventor of electron transfer dissociation and is a consultant for Thermo Fisher Scientific. The other authors declare no competing interests.
Figures



Similar articles
-
Electron-Transfer/Higher-Energy Collision Dissociation (EThcD)-Enabled Intact Glycopeptide/Glycoproteome Characterization.J Am Soc Mass Spectrom. 2017 Sep;28(9):1751-1764. doi: 10.1007/s13361-017-1701-4. Epub 2017 Jul 10. J Am Soc Mass Spectrom. 2017. PMID: 28695533 Free PMC article.
-
Site-specific glycan-peptide analysis for determination of N-glycoproteome heterogeneity.J Proteome Res. 2013 Dec 6;12(12):5791-800. doi: 10.1021/pr400783j. Epub 2013 Nov 1. J Proteome Res. 2013. PMID: 24090084
-
Glycoproteomic Alterations in Drug-Resistant Nonsmall Cell Lung Cancer Cells Revealed by Lectin Magnetic Nanoprobe-Based Mass Spectrometry.J Proteome Res. 2018 Nov 2;17(11):3761-3773. doi: 10.1021/acs.jproteome.8b00433. Epub 2018 Oct 17. J Proteome Res. 2018. PMID: 30261726
-
Arraying the post-translational glycoproteome (PTG).Curr Opin Chem Biol. 2014 Feb;18:62-9. doi: 10.1016/j.cbpa.2014.01.002. Epub 2014 Jan 30. Curr Opin Chem Biol. 2014. PMID: 24487061 Review.
-
Mass Spectrometry-Based Chemical and Enzymatic Methods for Global Analysis of Protein Glycosylation.Acc Chem Res. 2018 Aug 21;51(8):1796-1806. doi: 10.1021/acs.accounts.8b00200. Epub 2018 Jul 16. Acc Chem Res. 2018. PMID: 30011186 Free PMC article. Review.
Cited by
-
Examining and Fine-tuning the Selection of Glycan Compositions with GlyConnect Compozitor.Mol Cell Proteomics. 2020 Oct;19(10):1602-1618. doi: 10.1074/mcp.RA120.002041. Epub 2020 Jul 7. Mol Cell Proteomics. 2020. PMID: 32636234 Free PMC article.
-
Measuring change in glycoprotein structure.Curr Opin Struct Biol. 2022 Jun;74:102371. doi: 10.1016/j.sbi.2022.102371. Epub 2022 Apr 19. Curr Opin Struct Biol. 2022. PMID: 35452871 Free PMC article. Review.
-
High-throughput site-specific N-glycoproteomics reveals glyco-signatures for liver disease diagnosis.Natl Sci Rev. 2022 Apr 5;10(1):nwac059. doi: 10.1093/nsr/nwac059. eCollection 2023 Jan. Natl Sci Rev. 2022. PMID: 36879659 Free PMC article.
-
Specific N-glycans regulate an extracellular adhesion complex during somatosensory dendrite patterning.EMBO Rep. 2022 Jul 5;23(7):e54163. doi: 10.15252/embr.202154163. Epub 2022 May 19. EMBO Rep. 2022. PMID: 35586945 Free PMC article.
-
The glycosylation in SARS-CoV-2 and its receptor ACE2.Signal Transduct Target Ther. 2021 Nov 15;6(1):396. doi: 10.1038/s41392-021-00809-8. Signal Transduct Target Ther. 2021. PMID: 34782609 Free PMC article. Review.
References
-
- Varki, A. et al. Essentials of Glycobiology. Essentials of Glycobiology (Cold Spring Harbor Laboratory Press, NY, 2015).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

