Neutrophil activation and NETosis are the major drivers of thrombosis in heparin-induced thrombocytopenia
- PMID: 30899022
- PMCID: PMC6428879
- DOI: 10.1038/s41467-019-09160-7
Neutrophil activation and NETosis are the major drivers of thrombosis in heparin-induced thrombocytopenia
Abstract
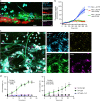
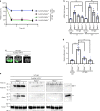
Heparin-induced thrombocytopenia/thrombosis (HIT) is a serious immune reaction to heparins, characterized by thrombocytopenia and often severe thrombosis with high morbidity and mortality. HIT is mediated by IgG antibodies against heparin/platelet factor 4 antigenic complexes. These complexes are thought to activate platelets leading to thrombocytopenia and thrombosis. Here we show that HIT immune complexes induce NETosis via interaction with FcγRIIa on neutrophils and through neutrophil-platelet association. HIT immune complexes induce formation of thrombi containing neutrophils, extracellular DNA, citrullinated histone H3 and platelets in a microfluidics system and in vivo, while neutrophil depletion abolishes thrombus formation. Absence of PAD4 or PAD4 inhibition with GSK484 abrogates thrombus formation but not thrombocytopenia, suggesting they are induced by separate mechanisms. NETs markers and neutrophils undergoing NETosis are present in HIT patients. Our findings demonstrating the involvement of NETosis in thrombosis will modify the current concept of HIT pathogenesis and may lead to new therapeutic strategies.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Warkentin, T. E. in Hematology 7th edn. (eds Hoffman, R. et al.) 1973–1983 (Elsevier, PA, 2018).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

