Targeting cellular metabolism to reduce head and neck cancer growth
- PMID: 30899051
- PMCID: PMC6428890
- DOI: 10.1038/s41598-019-41523-4
Targeting cellular metabolism to reduce head and neck cancer growth
Abstract
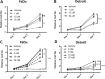
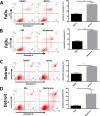
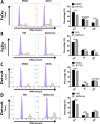
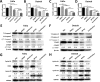
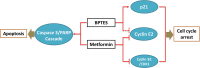
Head and neck squamous cell carcinoma (HNSCC) presents a major public health concern because of delayed diagnosis and poor prognosis. Malignant cells often reprogram their metabolism in order to promote their survival and proliferation. Aberrant glutaminase 1 (GLS1) expression enables malignant cells to undergo increased glutaminolysis and utilization of glutamine as an alternative nutrient. In this study, we found a significantly elevated GLS1 expression in HNSCC, and patients with high expression levels of GLS1 experienced shorter disease-free periods after therapy. We hypothesized that the GLS1 selective inhibitor, bis-2-(5-phenylacetamido-1,3,4-thiadiazol-2-yl)ethyl sulfide (BPTES), which curtails cells' glutamine consumption, may inhibit HNSCC cell growth. Our results support the idea that BPTES inhibits HNSCC growth by inducing apoptosis and cell cycle arrest. Considering that metformin can reduce glucose consumption, we speculated that metformin would enhance the anti-neoplasia effect of BPTES by suppressing malignant cells' glucose utilization. The combination of both compounds exhibited an additive inhibitory effect on cancer cell survival and proliferation. All of our data suggest that GLS1 is a promising therapeutic target for HNSCC treatment. Combining BPTES with metformin might achieve improved anti-cancer effects in HNSSC, which sheds light on using novel therapeutic strategies by dually targeting cellular metabolism.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

