IL-27 promotes NK cell effector functions via Maf-Nrf2 pathway during influenza infection
- PMID: 30899058
- PMCID: PMC6428861
- DOI: 10.1038/s41598-019-41478-6
IL-27 promotes NK cell effector functions via Maf-Nrf2 pathway during influenza infection
Abstract
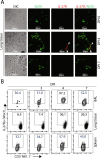
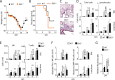
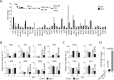
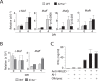
Influenza virus targets epithelial cells in the upper respiratory tract. Natural Killer (NK) cell-mediated early innate defense responses to influenza infection include the killing of infected epithelial cells and generation of anti-viral cytokines including interferon gamma (IFN-γ). To date, it is unclear how the underlying cytokine milieu during infection regulates NK cell effector functions. Our data show during influenza infection myeloid cell-derived IL-27 regulates the early-phase effector functions of NK cells in the bronchioalveolar and lung tissue. Lack of IL-27R (Il27ra-/-) or IL-27 (Ebi3-/-) resulted in impaired NK cell effector functions including the generation of anti-viral IFN-γ responses. We identify CD27+CD11b+ NK cells as the primary subset that expresses IL-27R, which predominantly produces IFN-γ within the upper respiratory tract of the infected mice. IL-27 alone was incapable of altering the effector functions of NK cells. However, IL-27 sensitizes NK cells to augment both in vitro and in vivo responses mediated via the NKG2D receptor. This 'priming' function of IL-27 is mediated partly via transcriptional pathways regulated by Mafs and Nrf2 transcriptionally regulating TFAM and CPT1. Our data for the first time establishes a novel role for IL-27 in regulating early-phase effector functions of NK cells during influenza infection.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Santoli D, Trinchieri G, Koprowski H. Cell-mediated cytotoxicity against virus-infected target cells in humans. II. Interferon induction and activation of natural killer cells. J Immunol. 1978;121:532–538. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

