An aquaporin mediates cell shape change required for cellular immunity in the beet armyworm, Spodoptera exigua
- PMID: 30899076
- PMCID: PMC6428837
- DOI: 10.1038/s41598-019-41541-2
An aquaporin mediates cell shape change required for cellular immunity in the beet armyworm, Spodoptera exigua
Abstract
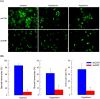
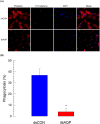
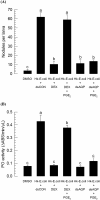
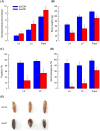
Cellular immunity in insects is accompanied by change in hemocyte shape. This study hypothesizes that cytoskeletal rearrangement is accompanied by transmembrane water transport to change cell volume, thus changing cell shape. A water-transporting pore (=aquaporin:AQP) has been identified in the beet armyworm, Spodoptera exigua. Its expression was detected in all developmental stages and tissues, although its transcription levels were different between biotic and abiotic conditions. Heterologous expression of Se-AQP in Sf9 cells showed that Se-AQP was localized on cell membrane. RNA interference (RNAi) using double-stranded RNA effectively suppressed its transcript levels. Under different ionic concentrations, hemocytes of RNAi-treated larvae did not change cell volume presumably due to malfunction in water transportation. Se-AQP might participate in glycerol transport because up-regulation of hemolymph glycerol titer after rapid cold-hardening was prevented by RNAi treatment against Se-AQP expression. The inhibitory effect of RNAi treatment on change of cell shape significantly impaired cellular immune responses such as phagocytosis and nodule formation upon bacterial challenge. RNAi treatment also significantly interfered with immature development of S. exigua. These results indicate that Se-AQP plays a crucial role in cell shape change that is required for cellular immunity and other physiological processes.
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
Aquaporins in Immune Cells and Inflammation: New Targets for Drug Development.Int J Mol Sci. 2021 Feb 12;22(4):1845. doi: 10.3390/ijms22041845. Int J Mol Sci. 2021. PMID: 33673336 Free PMC article. Review.
-
Role of a small G protein Ras in cellular immune response of the beet armyworm, Spodoptera exigua.J Insect Physiol. 2011 Mar;57(3):356-62. doi: 10.1016/j.jinsphys.2010.12.003. Epub 2010 Dec 15. J Insect Physiol. 2011. PMID: 21167168
-
PGE2 mediates cytoskeletal rearrangement of hemocytes via Cdc42, a small G protein, to activate actin-remodeling factors in Spodoptera exigua (Lepidoptera: Noctuidae).Arch Insect Biochem Physiol. 2019 Dec;102(4):e21607. doi: 10.1002/arch.21607. Epub 2019 Jul 23. Arch Insect Biochem Physiol. 2019. PMID: 31338878
-
EpOMEs act as immune suppressors in a lepidopteran insect, Spodoptera exigua.Sci Rep. 2020 Nov 19;10(1):20183. doi: 10.1038/s41598-020-77325-2. Sci Rep. 2020. PMID: 33214688 Free PMC article.
-
Overexpression of PGE2 synthase by in vivo transient expression enhances immunocompetency along with fitness cost in a lepidopteran insect.J Exp Biol. 2019 Jul 23;222(Pt 14):jeb207019. doi: 10.1242/jeb.207019. J Exp Biol. 2019. PMID: 31278129
Cited by
-
Identification and characterization of cold-responsive aquaporins from the larvae of a crambid pest Agriphila aeneociliella (Eversmann) (Lepidoptera: Crambidae).PeerJ. 2023 Nov 13;11:e16403. doi: 10.7717/peerj.16403. eCollection 2023. PeerJ. 2023. PMID: 38025732 Free PMC article.
-
Dual Oxidase-Derived Reactive Oxygen Species Against Bacillus thuringiensis and Its Suppression by Eicosanoid Biosynthesis Inhibitors.Front Microbiol. 2020 Mar 27;11:528. doi: 10.3389/fmicb.2020.00528. eCollection 2020. Front Microbiol. 2020. PMID: 32292400 Free PMC article.
-
Osmosis as nature's method for establishing optical alignment.Curr Biol. 2024 Apr 8;34(7):1569-1575.e3. doi: 10.1016/j.cub.2024.02.052. Epub 2024 Mar 20. Curr Biol. 2024. PMID: 38513653 Free PMC article.
-
Morphological and Chemical Changes in the Hemolymph of the Wax Moth Galleria mellonella Infected by the Entomopathogenic Fungus Conidiobolus coronatus.Pathogens. 2025 Jan 7;14(1):38. doi: 10.3390/pathogens14010038. Pathogens. 2025. PMID: 39860999 Free PMC article.
-
Aquaporins in Immune Cells and Inflammation: New Targets for Drug Development.Int J Mol Sci. 2021 Feb 12;22(4):1845. doi: 10.3390/ijms22041845. Int J Mol Sci. 2021. PMID: 33673336 Free PMC article. Review.
References
-
- Agre P, Saboori AM, Asimos A, Smith BL. Purification and partial characterization of the Mr 30,000 integral membrane protein associated with the erythrocyte Rh(D) antigen. J. Biol. Chem. 1987;262:17497–17503. - PubMed
-
- van Hoek AN, et al. Functional unit of 30 kDa for proximal tubule water channels as revealed by radiation inactivation. J. Biol. Chem. 1991;266:16633–16635. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

