Evolutionary-Conserved Allosteric Properties of Three Neuronal Calcium Sensor Proteins
- PMID: 30899213
- PMCID: PMC6417375
- DOI: 10.3389/fnmol.2019.00050
Evolutionary-Conserved Allosteric Properties of Three Neuronal Calcium Sensor Proteins
Abstract
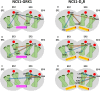
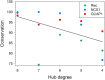
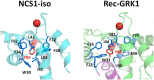
Neuronal Calcium Sensors (NCS) are highly conserved proteins specifically expressed in neurons. Calcium (Ca2+)-binding to their EF-hand motifs results in a conformational change, which is crucial for the recognition of a specific target and the downstream biological process. Here we present a comprehensive analysis of the allosteric communication between Ca2+-binding sites and the target interfaces of three NCS, namely NCS1, recoverin (Rec), and GCAP1. In particular, Rec was investigated in different Ca2+-loading states and in complex with a peptide from the Rhodopsin Kinase (GRK1) while NCS1 was studied in a Ca2+-loaded state in complex with either the same GRK1 target or a peptide from the D2 Dopamine receptor. A Protein Structure Network (PSN) accounting for persistent non-covalent interactions between amino acids was built for each protein state based on exhaustive Molecular Dynamics simulations. Structural network analysis helped unveiling the role of key amino acids in allosteric mechanisms and their evolutionary conservation among homologous proteins. Results for NCS1 highlighted allosteric inter-domain interactions between Ca2+-binding motifs and residues involved in target recognition. Robust long range, allosteric protein-target interactions were found also in Rec, in particular originating from the EF3 motif. Interestingly, Tyr 86, involved the hydrophobic packing of the N-terminal domain, was found to be a key residue for both intra- and inter-molecular communication with EF3, regardless of the presence of target or Ca2+ ions. Finally, based on a comprehensive topological PSN analysis for Rec, NCS1, and GCAP1 and multiple sequence alignments with homolog proteins, we propose that an evolution-driven correlation may exist between the amino acids mediating the highest number of persistent interactions (high-degree hubs) and their conservation. Such conservation is apparently fundamental for the specific structural dynamics required in signaling events.
Keywords: GCAP1; NCS1; molecular dynamics; neuronal calcium sensors; protein structure network; recoverin.
Figures


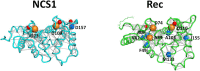

Similar articles
-
Effects of Membrane and Biological Target on the Structural and Allosteric Properties of Recoverin: A Computational Approach.Int J Mol Sci. 2019 Oct 10;20(20):5009. doi: 10.3390/ijms20205009. Int J Mol Sci. 2019. PMID: 31658639 Free PMC article.
-
Photoreceptor calcium sensor proteins in detergent-resistant membrane rafts are regulated via binding to caveolin-1.Cell Calcium. 2018 Jul;73:55-69. doi: 10.1016/j.ceca.2018.04.003. Epub 2018 Apr 14. Cell Calcium. 2018. PMID: 29684785
-
Molecular structure and target recognition of neuronal calcium sensor proteins.Front Mol Neurosci. 2012 Feb 9;5:10. doi: 10.3389/fnmol.2012.00010. eCollection 2012 Jan 19. Front Mol Neurosci. 2012. Retraction in: Front Mol Neurosci. 2016 May 20;9:38. doi: 10.3389/fnmol.2016.00038. PMID: 22363261 Free PMC article. Retracted.
-
Molecular structure and target recognition of neuronal calcium sensor proteins.Biochim Biophys Acta. 2012 Aug;1820(8):1205-13. doi: 10.1016/j.bbagen.2011.10.003. Epub 2011 Oct 13. Biochim Biophys Acta. 2012. PMID: 22020049 Free PMC article. Review.
-
Dimerization of Neuronal Calcium Sensor Proteins.Front Mol Neurosci. 2018 Nov 2;11:397. doi: 10.3389/fnmol.2018.00397. eCollection 2018. Front Mol Neurosci. 2018. PMID: 30450035 Free PMC article. Review.
Cited by
-
Modulation of Guanylate Cyclase Activating Protein 1 (GCAP1) Dimeric Assembly by Ca2+ or Mg2+: Hints to Understand Protein Activity.Biomolecules. 2020 Oct 5;10(10):1408. doi: 10.3390/biom10101408. Biomolecules. 2020. PMID: 33027977 Free PMC article.
-
Novel dual-pathogen multi-epitope mRNA vaccine development for Brucella melitensis and Mycobacterium tuberculosis in silico approach.PLoS One. 2024 Oct 28;19(10):e0309560. doi: 10.1371/journal.pone.0309560. eCollection 2024. PLoS One. 2024. PMID: 39466745 Free PMC article.
-
Molecular Properties of Human Guanylate Cyclase-Activating Protein 3 (GCAP3) and Its Possible Association with Retinitis Pigmentosa.Int J Mol Sci. 2022 Mar 17;23(6):3240. doi: 10.3390/ijms23063240. Int J Mol Sci. 2022. PMID: 35328663 Free PMC article.
-
Alterations in calmodulin-cardiac ryanodine receptor molecular recognition in congenital arrhythmias.Cell Mol Life Sci. 2022 Feb 8;79(2):127. doi: 10.1007/s00018-022-04165-w. Cell Mol Life Sci. 2022. PMID: 35133504 Free PMC article.
-
Protein conformational switch discerned via network centrality properties.Comput Struct Biotechnol J. 2021 Jun 5;19:3599-3608. doi: 10.1016/j.csbj.2021.06.004. eCollection 2021. Comput Struct Biotechnol J. 2021. PMID: 34257839 Free PMC article.
References
-
- Abraham M. J., Murtola T., Schulz R., Páll S., Smith J. C., Hess B., et al. (2015). GROMACS: high performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 1, 19–25. 10.1016/j.softx.2015.06.001 - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

