Bioconversion of Beet Molasses to Alpha-Galactosidase and Ethanol
- PMID: 30899250
- PMCID: PMC6416216
- DOI: 10.3389/fmicb.2019.00405
Bioconversion of Beet Molasses to Alpha-Galactosidase and Ethanol
Abstract
Molasses are sub-products of the sugar industry, rich in sucrose and containing other sugars like raffinose, glucose, and fructose. Alpha-galactosidases (EC. 3.2.1.22) catalyze the hydrolysis of alpha-(1,6) bonds of galactose residues in galacto-oligosaccharides (melibiose, raffinose, and stachyose) and complex galactomannans. Alpha-galactosidases have important applications, mainly in the food industry but also in the pharmaceutical and bioenergy sectors. However, the cost of the enzyme limits the profitability of most of these applications. The use of cheap sub-products, such as molasses, as substrates for production of alpha-galactosidases, reduces the cost of the enzymes and contributes to the circular economy. Alpha-galactosidase is a specially indicated bioproduct since, at the same time, it allows to use the raffinose present in molasses. This work describes the development of a two-step system for the valuation of beet molasses, based on their use as substrate for alpha-galactosidase and bioethanol production by Saccharomyces cerevisiae. Since this yeast secretes high amounts of invertase, to avoid congest the secretory route and to facilitate alpha-galactosidase purification from the culture medium, a mutant in the SUC2 gene (encoding invertase) was constructed. After a statistical optimization of culture conditions, this mutant yielded a very high rate of molasses bioconversion to alpha-galactosidase. In the second step, the SUC2 wild type yeast strain fermented the remaining sucrose to ethanol. A procedure to recycle the yeast biomass, by using it as nitrogen source to supplement molasses, was also developed.
Keywords: Saccharomyces cerevisiae; alpha-galactosidase; beet molasses; bioconversion; ethanol; invertase.
Figures
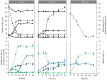

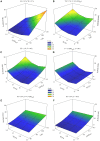
Similar articles
-
Valuation of agro-industrial wastes as substrates for heterologous production of α-galactosidase.Microb Cell Fact. 2018 Sep 3;17(1):137. doi: 10.1186/s12934-018-0988-6. Microb Cell Fact. 2018. PMID: 30176892 Free PMC article.
-
Optimization of Saccharomyces cerevisiae α-galactosidase production and application in the degradation of raffinose family oligosaccharides.Microb Cell Fact. 2019 Oct 10;18(1):172. doi: 10.1186/s12934-019-1222-x. Microb Cell Fact. 2019. PMID: 31601209 Free PMC article.
-
Derepression of galactose metabolism in melibiase producing bakers' and distillers' yeast.J Biotechnol. 1999 Jun 11;72(1-2):213-28. doi: 10.1016/s0168-1656(99)00108-x. J Biotechnol. 1999. PMID: 12680392
-
Microbial α-galactosidases: Efficient biocatalysts for bioprocess technology.Bioresour Technol. 2022 Jan;344(Pt B):126293. doi: 10.1016/j.biortech.2021.126293. Epub 2021 Nov 6. Bioresour Technol. 2022. PMID: 34752888 Review.
-
Microbial production and biotechnological applications of α-galactosidase.Int J Biol Macromol. 2020 May 1;150:1294-1313. doi: 10.1016/j.ijbiomac.2019.10.140. Epub 2019 Nov 17. Int J Biol Macromol. 2020. PMID: 31747573 Review.
Cited by
-
Molecular advances in microbial α-galactosidases: challenges and prospects.World J Microbiol Biotechnol. 2022 Jul 1;38(9):148. doi: 10.1007/s11274-022-03340-2. World J Microbiol Biotechnol. 2022. PMID: 35773364 Review.
-
Rational engineering of the Trichoderma reesei RUT-C30 strain into an industrially relevant platform for cellulase production.Biotechnol Biofuels. 2020 May 22;13:93. doi: 10.1186/s13068-020-01732-w. eCollection 2020. Biotechnol Biofuels. 2020. PMID: 32461765 Free PMC article.
-
Evaluation of the Impact of an Enzymatic Preparation Catalyzing the Decomposition of Raffinose from Poor-Quality Beets during the White Sugar Production Process.Molecules. 2024 Jul 26;29(15):3526. doi: 10.3390/molecules29153526. Molecules. 2024. PMID: 39124931 Free PMC article.
-
Direct Isomaltulose Synthesis From Beet Molasses by Immobilized Sucrose Isomerase.Front Bioeng Biotechnol. 2021 Jul 16;9:691547. doi: 10.3389/fbioe.2021.691547. eCollection 2021. Front Bioeng Biotechnol. 2021. PMID: 34336804 Free PMC article.
-
Comprehensive Studies of the Processes of the Molecular Transfer of the Momentum, Thermal Energy and Mass in the Nutrient Media of Biotechnological Industries.Bioengineering (Basel). 2022 Jan 6;9(1):18. doi: 10.3390/bioengineering9010018. Bioengineering (Basel). 2022. PMID: 35049728 Free PMC article.
References
-
- Álvarez-Cao M.-E. (2017). Optimización de la Producción Heteróloga de la Enzima α-Galactosidasa de Saccharomyces cerevisiae a Partir de Residuos Agroindustriales. Ph.D. thesis, Universidade da Coruña. Available online at: https://ruc.udc.es/dspace/handle/2183/19936
-
- Anisha G. S. (2017). α-Galactosidases, in Current Developments in Biotechnology and Bioengineering: Production, Isolation and Purification of Industrial Products, eds Pandey A., Negi S., Soccol C. (Amsterdam: Elsevier B.V.), 369–394. 10.1016/B978-0-444-63662-1.00016-6 - DOI
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

