Loss of Jak2 protects cardiac allografts from chronic rejection by attenuating Th1 response along with increased regulatory T cells
- PMID: 30899367
- PMCID: PMC6413256
Loss of Jak2 protects cardiac allografts from chronic rejection by attenuating Th1 response along with increased regulatory T cells
Abstract
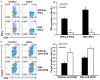
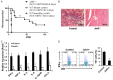
Chronic rejection acts as the most formidable obstacle for organ transplantation in clinical settings. Herein we demonstrated in a cardiac transplantation model that blockade of Janus kinase 2 (Jak2) provides protection for cardiac allografts against chronic rejection. Specifically, loss of Jak2 almost completely abolished the production of IFN-γ+ Th1 cells, while the percentage of Foxp3+ regulatory T cells (Tregs) was significantly increased. As a result, loss of Jak2 significantly prolonged allograft survival (58 ± 30.6 days vs. 7 ± 0.3 days). Particularly, 4 out of 13 Jak2 deficient recipients (30%) showed long-term acceptance of allografts as manifested by the graft survival time > 100 days. Cellular studies revealed that Jak2 deficiency did not impact the intrinsic proliferative capability for CD4+ T cells in response to nonspecific polyclonal and allogenic stimulation. Mechanistic studies documented that the impaired Th1 development was caused by the attenuated IFN-γ/STAT1 and IL-12/STAT4 signaling along with repressed expression of Th1 transcription factors T-bet, Hlx and Runx3. However, the IL-2/STAT5 signaling remained intact, which ensured normal Treg development in Jak2-/- naïve CD4 T cells. Together, our data support that blockade of Jak2 may have therapeutic potential for prevention and treatment of allograft rejection in clinical settings.
Keywords: Jak2; allograft; cardiac transplantation; chronic rejection; regulatory T cells.
Conflict of interest statement
None.
Figures





References
-
- Schliesser U, Streitz M, Sawitzki B. Tregs: application for solid-organ transplantation. Curr Opin Organ Transplant. 2012;17:34–41. - PubMed
-
- Newell KA, Phippard D, Turka LA. Regulatory cells and cell signatures in clinical transplantation tolerance. Curr Opin Immunol. 2011;23:655–659. - PubMed
-
- Bluestone JA. Regulatory T-cell therapy: is it ready for the clinic? Nat Rev Immunol. 2005;5:343–349. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
