Super-enhancers: novel target for pancreatic ductal adenocarcinoma
- PMID: 30899425
- PMCID: PMC6422180
- DOI: 10.18632/oncotarget.26704
Super-enhancers: novel target for pancreatic ductal adenocarcinoma
Abstract
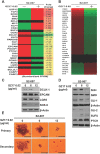
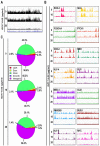
Super-enhancers (SEs) are unique areas of the genome which drive high-level of transcription and play a pivotal role in the cell physiology. Previous studies have established several important genes in cancer as SE-driven oncogenes. It is likely that oncogenes may hack the resident tissue regenerative program and interfere with SE-driven repair networks, leading to the specific pancreatic ductal adenocarcinoma (PDAC) phenotype. Here, we used ChIP-Seq to identify the presence of SE in PDAC cell lines. Differential H3K27AC marks were identified at enhancer regions of genes including c-MYC, MED1, OCT-4, NANOG, and SOX2 that can act as SE in non-cancerous, cancerous and metastatic PDAC cell lines. GZ17-6.02 affects acetylation of the genes, reduces transcription of major transcription factors, sonic hedgehog pathway proteins, and stem cell markers. In accordance with the decrease in Oct-4 expression, ChIP-Seq revealed a significant decrease in the occupancy of OCT-4 in the entire genome after GZ17-6.02 treatment suggesting the possible inhibitory effect of GZ17-6.02 on PDAC. Hence, SE genes are associated with PDAC and targeting their regulation with GZ17-6.02 offers a novel approach for treatment.
Keywords: ChIP-Seq; PDAC; cancer stem cells; super-enhancers; therapeutics.
Conflict of interest statement
CONFLICTS OF INTEREST Dr. Animesh Dhar is a consultant of Genzada Pharmaceuticals and Dr. Cameron West is employed by Genzada Pharmaceuticals. Other authors have no conflicts of interest.
Figures




Similar articles
-
Super-enhancer-driven TOX2 mediates oncogenesis in Natural Killer/T Cell Lymphoma.Mol Cancer. 2023 Apr 10;22(1):69. doi: 10.1186/s12943-023-01767-1. Mol Cancer. 2023. PMID: 37032358 Free PMC article.
-
Re-engineering the Pancreas Tumor Microenvironment: A "Regenerative Program" Hacked.Clin Cancer Res. 2017 Apr 1;23(7):1647-1655. doi: 10.1158/1078-0432.CCR-16-3275. Clin Cancer Res. 2017. PMID: 28373363 Free PMC article. Review.
-
Targeting Super-Enhancers via Nanoparticle-Facilitated BRD4 and CDK7 Inhibitors Synergistically Suppresses Pancreatic Ductal Adenocarcinoma.Adv Sci (Weinh). 2020 Feb 16;7(7):1902926. doi: 10.1002/advs.201902926. eCollection 2020 Apr. Adv Sci (Weinh). 2020. PMID: 32274304 Free PMC article.
-
Triptolide targets super-enhancer networks in pancreatic cancer cells and cancer-associated fibroblasts.Oncogenesis. 2020 Nov 9;9(11):100. doi: 10.1038/s41389-020-00285-9. Oncogenesis. 2020. PMID: 33168807 Free PMC article.
-
Super-enhancers: critical roles and therapeutic targets in hematologic malignancies.J Hematol Oncol. 2019 Jul 16;12(1):77. doi: 10.1186/s13045-019-0757-y. J Hematol Oncol. 2019. PMID: 31311566 Free PMC article. Review.
Cited by
-
Topical GZ21T Inhibits the Growth of Actinic Keratoses in a UVB-Induced Model of Skin Carcinogenesis.JID Innov. 2023 May 6;3(4):100206. doi: 10.1016/j.xjidi.2023.100206. eCollection 2023 Jul. JID Innov. 2023. PMID: 37533581 Free PMC article.
-
GZ17-6.02 Inhibits the Growth of EGFRvIII+ Glioblastoma.Int J Mol Sci. 2022 Apr 10;23(8):4174. doi: 10.3390/ijms23084174. Int J Mol Sci. 2022. PMID: 35456993 Free PMC article.
-
GZ17-6.02 interacts with proteasome inhibitors to kill multiple myeloma cells.Oncotarget. 2024 Mar 5;15:159-174. doi: 10.18632/oncotarget.28558. Oncotarget. 2024. PMID: 38441437 Free PMC article.
-
MICAL2 Promotes Pancreatic Cancer Growth and Metastasis.Cancer Res. 2025 Mar 14;85(6):1049-1063. doi: 10.1158/0008-5472.CAN-24-0744. Cancer Res. 2025. PMID: 39745352 Free PMC article.
-
Super-Enhancers, Phase-Separated Condensates, and 3D Genome Organization in Cancer.Cancers (Basel). 2022 Jun 10;14(12):2866. doi: 10.3390/cancers14122866. Cancers (Basel). 2022. PMID: 35740532 Free PMC article. Review.
References
-
- Burris HA, 3rd, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo AM, Tarassoff P, Nelson R, Dorr FA, Stephens CD, Von Hoff DD. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403–13. doi: 10.1200/JCO.1997.15.6.2403. - DOI - PubMed
-
- Ponnurangam S, Dandawate PR, Dhar A, Tawfik OW, Parab RR, Mishra PD, Ranadive P, Sharma R, Mahajan G, Umar S, Weir SJ, Sugumar A, Jensen RA, et al. Quinomycin A targets Notch signaling pathway in pancreatic cancer stem cells. Oncotarget. 2016;7:3217–32. doi: 10.18632/oncotarget.6560. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

