Oral administration of the cannabigerol derivative VCE-003.2 promotes subventricular zone neurogenesis and protects against mutant huntingtin-induced neurodegeneration
- PMID: 30899454
- PMCID: PMC6407204
- DOI: 10.1186/s40035-019-0148-x
Oral administration of the cannabigerol derivative VCE-003.2 promotes subventricular zone neurogenesis and protects against mutant huntingtin-induced neurodegeneration
Abstract
Background: The administration of certain cannabinoids provides neuroprotection in models of neurodegenerative diseases by acting through various cellular and molecular mechanisms. Many cannabinoid actions in the nervous system are mediated by CB1 receptors, which can elicit psychotropic effects, but other targets devoid of psychotropic activity, including CB2 and nuclear PPARγ receptors, can also be the target of specific cannabinoids.
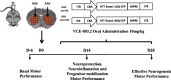
Methods: We investigated the pro-neurogenic potential of the synthetic cannabigerol derivative, VCE-003.2, in striatal neurodegeneration by using adeno-associated viral expression of mutant huntingtin in vivo and mouse embryonic stem cell differentiation in vitro.
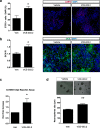
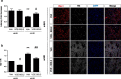
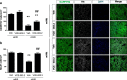
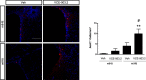
Results: Oral administration of VCE-003.2 protected striatal medium spiny neurons from mutant huntingtin-induced damage, attenuated neuroinflammation and improved motor performance. VCE-003.2 bioavailability was characterized and the potential undesired side effects were evaluated by analyzing hepatotoxicity after chronic treatment. VCE-003.2 promoted subventricular zone progenitor mobilization, increased doublecortin-positive migrating neuroblasts towards the injured area, and enhanced effective neurogenesis. Moreover, we demonstrated the proneurogenic activity of VCE-003.2 in embryonic stem cells. VCE-003.2 was able to increase neuroblast formation and striatal-like CTIP2-mediated neurogenesis.
Conclusions: The cannabigerol derivative VCE-003.2 improves subventricular zone-derived neurogenesis in response to mutant huntingtin-induced neurodegeneration, and is neuroprotective by oral administration.
Keywords: Cannabinoid; Huntingtin; Neurodegeneration; Neurogenesis; PPAR.
Conflict of interest statement
Animal studies were approved by ethics committee of Complutense University and Cordoba University (Spain). Consent to participate is not applicable.Not applicable.EM is scientific advisors of Emerald Health Pharmaceuticals.
Figures


References
-
- Ross CA, Aylward EH, Wild EJ, Langbehn DR, Long JD, Warner JH, Scahill RI, Leavitt BR, Stout JC, Paulsen JS, Reilmann R, Unschuld PG, Wexler A, Margolis RL, Tabrizi SJ. Huntington disease: Natural history, biomarkers and prospects for therapeutics. Nat Rev Neurol. 2014;10:204–216. doi: 10.1038/nrneurol.2014.24. - DOI - PubMed
-
- Conforti P, Besusso D, Bocchi VD, Faedo A, Cesana E, Rossetti G, Ranzani V, Svendsen CN, Thompson LM, Toselli M, Biella G, Pagani M, Cattaneo E. Faulty neuronal determination and cell polarization are reverted by modulating HD early phenotypes. Proc Natl Acad Sci U S A. 2018;115:E762–E771. doi: 10.1073/pnas.1715865115. - DOI - PMC - PubMed
-
- Lim RG, Salazar LL, Wilton DK, King AR, Stocksdale JT, Sharifabad D, Lau AL, Stevens B, Reidling JC, Winokur ST, Casale MS, Thompson LM, Pardo M, AGG D-B, Straccia M, Sanders P, Alberch J, Canals JM, Kaye JA, Dunlap M, Jo L, May H, Mount E, Anderson-Bergman C, Haston K, Finkbeiner S, Kedaigle AJ, Gipson TA, Yildirim F, Ng CW, Milani P, Housman DE, Fraenkel E, Allen ND, Kemp PJ, Atwal RS, Biagioli M, Gusella JF, ME MD, Akimov SS, Arbez N, Stewart J, Ross CA, Mattis VB, Tom CM, Ornelas L, Sahabian A, Lenaeus L, Mandefro B, Sareen D, Svendsen CN. Developmental alterations in Huntington’s disease neural cells and pharmacological rescue in cells and mice. Nat Neurosci. 2017;20:648–660. doi: 10.1038/nn.4532. - DOI - PMC - PubMed
-
- Godin JD, Colombo K, Molina-Calavita M, Keryer G, Zala D, Charrin BC, Dietrich P, Volvert M-L, Guillemot F, Dragatsis I, Bellaiche Y, Saudou F, Nguyen L, Humbert S. Huntingtin Is Required for Mitotic Spindle Orientation and Mammalian Neurogenesis. Neuron. 2010;67:392–406. doi: 10.1016/j.neuron.2010.06.027. - DOI - PubMed

