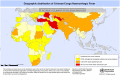
Diagnostic tests for Crimean-Congo haemorrhagic fever: a widespread tickborne disease
- PMID: 30899574
- PMCID: PMC6407549
- DOI: 10.1136/bmjgh-2018-001114
Diagnostic tests for Crimean-Congo haemorrhagic fever: a widespread tickborne disease
Abstract
Crimean-Congo haemorrhagic fever (CCHF) is a widespread tickborne disease that circulates in wild and domestic animal hosts, and causes severe and often fatal haemorrhagic fever in infected humans. Due to the lack of treatment options or vaccines, and a high fatality rate, CCHF virus (CCHFV) is considered a high-priority pathogen according to the WHO R&D Blueprint. Several commercial reverse transcriptase PCR (RT-PCR) and serological diagnostic assays for CCHFV are already available, including febrile agent panels to distinguish CCHFV from other viral haemorrhagic fever agents; however, the majority of international laboratories use inhouse assays. As CCHFV has numerous amplifying animal hosts, a cross-sectoral 'One Health' approach to outbreak prevention is recommended to enhance notifications and enable early warning for genetic and epidemiological shifts in the human, animal and tick populations. However, a lack of guidance for surveillance in animals, harmonisation of case identification and validated serodiagnostic kits for animal testing hinders efforts to strengthen surveillance systems. Additionally, as RT-PCR tests tend to be lineage-specific for regional circulating strains, there is a need for pan-lineage sensitive diagnostics. Adaptation of existing tests to point-of-care molecular diagnostic platforms that can be implemented in clinic or field-based settings would be of value given the potential for CCHFV outbreaks in remote or low-resource areas. Finally, improved access to clinical specimens for validation of diagnostics would help to accelerate development of new tests. These gaps should be addressed by updated target product profiles for CCHFV diagnostics.
Keywords: CCHF; crimean-congo haemorrhagic fever; in vitro diagnostics; outbreak.
Conflict of interest statement
Competing interests: None declared.
Figures
References
-
- CDC , 2017. Crimean-Congo Hemorrhagic Fever (CCHF).. Available from: https://www.cdc.gov/vhf/crimean-congo/index.html [Accessed 12 Oct 2017].
-
- WHO , 2017. a. Crimean-Congo haemorrhagic fever (CCHF).. Available from: http://www.who.int/csr/disease/crimean_congoHF/en/ [Accessed 12 Oct 2017].
-
- WHO , 2017. R&D Blueprint for action to prevent epidemics.. Available from: http://www.who.int/blueprint/en/ [Accessed 12 Sep 2017].
-
- Chinikar S, Mirahmadi R, Moradi M. Crimean-Congo Hemorrhagic Fever (CCHF) : Lorenzo-Morales J, Zoonosis. IntechOpen, 2012:193–212. 10.5772/2125 - DOI
LinkOut - more resources
Full Text Sources
Research Materials