Similarities Between Embryo Development and Cancer Process Suggest New Strategies for Research and Therapy of Tumors: A New Point of View
- PMID: 30899759
- PMCID: PMC6416183
- DOI: 10.3389/fcell.2019.00020
Similarities Between Embryo Development and Cancer Process Suggest New Strategies for Research and Therapy of Tumors: A New Point of View
Abstract
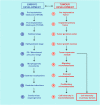
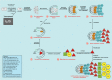
Here, I propose that cancer stem cells (CSCs) would be equivalent to para-embryonic stem cells (p-ESCs), derived from adult cells de-re-programmed to a ground state. p-ESCs would differ from ESCs by the absence of genomic homeostasis. A p-ESC would constitute the cancer cell of origin (i-CSC or CSC0), capable of generating an initial tumor, corresponding to a pre-implantation blastocyst. In a niche with proper signals, it would engraft as a primary tumor, corresponding to a post-implantation blastocyst. i-CSC progeny would form primary pluripotent and slow self-renewing CSCs (CSC1s), blocked in an undifferentiated state, corresponding to epiblast cells; CSC1s would be tumor-initiating cells (TICs). CSC1s would generate secondary CSCs (CSC2s), corresponding to hypoblast cells; CSC2s would be tumor growth cells (TGCs). CSC1s/CSC2s would generate tertiary CSCs (CSC3s), with a mesenchymal phenotype; CSC3s would be tumor migrating cells (TMCs), corresponding to mesodermal precursors at primitive streak. CSC3s with more favorable conditions (normoxia), by asymmetrical division, would differentiate into cancer progenitor cells (CPCs), and these into cancer differentiated cells (CDCs), thus generating a defined cell hierarchy and tumor progression, mimicking somito-histo-organogenesis. CSC3s with less favorable conditions (hypoxia) would delaminate and migrate as quiescent circulating micro-metastases, mimicking mesenchymal cells in gastrula morphogenetic movements. In metastatic niches, these CSC3s would install and remain dormant in the presence of epithelial/mesenchymal transition (EMT) signals and hypoxia. But, in the presence of mesenchymal/epithelial transition (MET) signals and normoxia, they would revert to self-renewing CSC1s, reproducing the same cell hierarchy of the primary tumor as macro-metastases. Further similarities between ontogenesis and oncogenesis involving crucial factors, such as ID, HSP70, HLA-G, CD44, LIF, and STAT3, are strongly evident at molecular, physiological and immunological levels. Much experimental data about these factors led to considering the cancer process as ectopic rudimentary ontogenesis, where CSCs have privileged immunological conditions. These would consent to CSC development in an adverse environment, just like an embryo, which is tolerated, accepted and favored by the maternal organism in spite of its paternal semi-allogeneicity. From all these considerations, novel research directions, potential innovative tumor therapy and prophylaxis strategies might, theoretically, result.
Keywords: CSCs; ESCs; HLA-G; HSP70; MSCs; tumor hierarchy/immunoevasion/therapy/prophylaxis.
Figures

References
-
- American Association for Cancer Research (2012). Leukemia Inhibitory Factor May be a Promising Target Against Pancreatic Cancer. Public Release. Available at: https://www.eurekalert.org/pub_releases/2012-06/aafc-lif061412.php
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

