Electrophysiological Properties of Medium Spiny Neuron Subtypes in the Caudate-Putamen of Prepubertal Male and Female Drd1a-tdTomato Line 6 BAC Transgenic Mice
- PMID: 30899778
- PMCID: PMC6426437
- DOI: 10.1523/ENEURO.0016-19.2019
Electrophysiological Properties of Medium Spiny Neuron Subtypes in the Caudate-Putamen of Prepubertal Male and Female Drd1a-tdTomato Line 6 BAC Transgenic Mice
Abstract
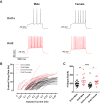
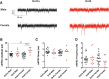
The caudate-putamen is a striatal brain region essential for sensorimotor behaviors, habit learning, and other cognitive and premotor functions. The output and predominant neuron of the caudate-putamen is the medium spiny neuron (MSN). MSNs present discrete cellular subtypes that show differences in neurochemistry, dopamine receptor expression, efferent targets, gene expression, functional roles, and most importantly for this study, electrophysiological properties. MSN subtypes include the striatonigral and the striatopallidal groups. Most studies identify the striatopallidal MSN subtype as being more excitable than the striatonigral MSN subtype. However, there is some divergence between studies regarding the exact differences in electrophysiological properties. Furthermore, MSN subtype electrophysiological properties have not been reported disaggregated by biological sex. We addressed these questions using prepubertal male and female Drd1a-tdTomato line 6 BAC transgenic mice, an important transgenic line that has not yet received extensive electrophysiological analysis. We made acute caudate-putamen brain slices and assessed a robust battery of 16 relevant electrophysiological properties using whole-cell patch-clamp recording, including intrinsic membrane, action potential, and miniature EPSC (mEPSC) properties. We found that: (1) MSN subtypes exhibited multiple differential electrophysiological properties in both sexes, including rheobase, action potential threshold and width, input resistance in both the linear and rectified ranges, and mEPSC amplitude; (2) select electrophysiological properties showed interactions between MSN subtype and sex. These findings provide a comprehensive evaluation of mouse caudate-putamen MSN subtype electrophysiological properties across females and males, both confirming and extending previous studies.
Keywords: caudate putamen; electrophysiology; intrinsic excitability; medium spiny neurons; rodent; sex differences.
Figures



Similar articles
-
Perinatal activation of ERα and ERβ but not GPER-1 masculinizes female rat caudate-putamen medium spiny neuron electrophysiological properties.J Neurophysiol. 2021 Jun 1;125(6):2322-2338. doi: 10.1152/jn.00063.2021. Epub 2021 May 12. J Neurophysiol. 2021. PMID: 33978486 Free PMC article.
-
Electrophysiological properties of medium spiny neurons in the nucleus accumbens core of prepubertal male and female Drd1a-tdTomato line 6 BAC transgenic mice.J Neurophysiol. 2018 Oct 1;120(4):1712-1727. doi: 10.1152/jn.00257.2018. Epub 2018 Jul 5. J Neurophysiol. 2018. PMID: 29975170 Free PMC article.
-
Intrinsic excitability varies by sex in prepubertal striatal medium spiny neurons.J Neurophysiol. 2015 Feb 1;113(3):720-9. doi: 10.1152/jn.00687.2014. Epub 2014 Nov 5. J Neurophysiol. 2015. PMID: 25376786 Free PMC article.
-
Sex Differences in Medium Spiny Neuron Excitability and Glutamatergic Synaptic Input: Heterogeneity Across Striatal Regions and Evidence for Estradiol-Dependent Sexual Differentiation.Front Endocrinol (Lausanne). 2018 Apr 18;9:173. doi: 10.3389/fendo.2018.00173. eCollection 2018. Front Endocrinol (Lausanne). 2018. PMID: 29720962 Free PMC article. Review.
-
Biological Sex, Estradiol and Striatal Medium Spiny Neuron Physiology: A Mini-Review.Front Cell Neurosci. 2018 Dec 12;12:492. doi: 10.3389/fncel.2018.00492. eCollection 2018. Front Cell Neurosci. 2018. PMID: 30618639 Free PMC article. Review.
Cited by
-
Direct neuronal conversion of microglia/macrophages reinstates neurological function after stroke.Proc Natl Acad Sci U S A. 2023 Oct 17;120(42):e2307972120. doi: 10.1073/pnas.2307972120. Epub 2023 Oct 10. Proc Natl Acad Sci U S A. 2023. PMID: 37812721 Free PMC article.
-
Perinatal activation of ERα and ERβ but not GPER-1 masculinizes female rat caudate-putamen medium spiny neuron electrophysiological properties.J Neurophysiol. 2021 Jun 1;125(6):2322-2338. doi: 10.1152/jn.00063.2021. Epub 2021 May 12. J Neurophysiol. 2021. PMID: 33978486 Free PMC article.
-
Estradiol rapidly modulates excitatory synapse properties in a sex- and region-specific manner in rat nucleus accumbens core and caudate-putamen.J Neurophysiol. 2019 Sep 1;122(3):1213-1225. doi: 10.1152/jn.00264.2019. Epub 2019 Jul 17. J Neurophysiol. 2019. PMID: 31314648 Free PMC article.
-
Morphine Differentially Alters the Synaptic and Intrinsic Properties of D1R- and D2R-Expressing Medium Spiny Neurons in the Nucleus Accumbens.Front Synaptic Neurosci. 2019 Dec 20;11:35. doi: 10.3389/fnsyn.2019.00035. eCollection 2019. Front Synaptic Neurosci. 2019. PMID: 31920618 Free PMC article.
-
Striatal Chloride Dysregulation and Impaired GABAergic Signaling Due to Cation-Chloride Cotransporter Dysfunction in Huntington's Disease.Front Cell Neurosci. 2022 Jan 14;15:817013. doi: 10.3389/fncel.2021.817013. eCollection 2021. Front Cell Neurosci. 2022. PMID: 35095429 Free PMC article. Review.
References
-
- Andersen SL, Rutstein M, Benzo JM, Hostetter JC, Teicher MH (1997) Sex differences in dopamine receptor overproduction and elimination. Neuroreport 8:1495–1498. - PubMed
-
- Arnauld E, Dufy B, Pestre M, Vincent JD (1981) Effects of estrogens on the responses of caudate neurons to microiontophoretically applied dopamine. Neurosci Lett 21:325–331. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
