Neuronal NO synthase mediates plenylephrine induced cardiomyocyte hypertrophy through facilitation of NFAT-dependent transcriptional activity
- PMID: 30899802
- PMCID: PMC6412025
- DOI: 10.1016/j.bbrep.2019.100620
Neuronal NO synthase mediates plenylephrine induced cardiomyocyte hypertrophy through facilitation of NFAT-dependent transcriptional activity
Abstract
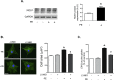
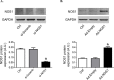
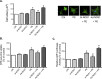
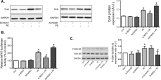
Neuronal nitric oxide synthase (NOS1) has been consistently shown to be the predominant isoform of NOS and/or NOS-derived NO that may be involved in the myocardial remodeling including cardiac hypertrophy. However, the direct functional contribution of NOS1 in this process remains to be elucidated. Therefore, in the present study, we attempted to use silent RNA and adenovirus mediated silencing or overexpression to investigate the role of NOS1 and the associated molecular signaling mechanisms during OKphenylephrine (PE)-induced cardiac hypertrophy growth in neonatal rat ventricular cardiomyocytes (NRVMs). We found that the expression of NOS1 was enhanced in PE-induced hypertrophic cardiomyocytes. Moreover, LVNIO treatment, a selective NOS1 inhibitor, significantly decreased PE-induced NRVMs hypertrophy and [3H]-leucine incorporation. We demonstrated that NOS1 gene silencing attenuated both the increased size and the transcriptional activity of the hypertrophic marker atrial natriuretic factor (ANF) induced by PE stimulation. Further investigation suggested that deficiency of NOS1-induced diminished NRVMS hypertrophy resulted in decreased calcineurin protein expression and activity (assessed by measuring the transcriptional activity of NFAT) and, an increased activity of the anti-hypertrophic pathway, GSK-3β (estimated by its augmented phosphorylated level). In contrast, exposing the NOS1 overexpressed NRVMs to PE-treatment further increased the hypertrophic growth, ANF transcriptional activity and calcineurin activity. Together, the results of the present study suggest that NOS1 is directly involved in controlling the development of cardiomyocyte hypertrophy.
Keywords: Adenovirus; Cardiomyocyte hypertrophy; Gene silencing; NFAT; Neuronal NO synthase.
Figures
Similar articles
-
GSK-3β/NFAT Signaling Is Involved in Testosterone-Induced Cardiac Myocyte Hypertrophy.PLoS One. 2016 Dec 15;11(12):e0168255. doi: 10.1371/journal.pone.0168255. eCollection 2016. PLoS One. 2016. PMID: 27977752 Free PMC article.
-
Integrin stimulation-induced hypertrophy in neonatal rat cardiomyocytes is NO-dependent.Mol Cell Biochem. 2009 Jan;320(1-2):75-84. doi: 10.1007/s11010-008-9900-8. Epub 2008 Aug 9. Mol Cell Biochem. 2009. PMID: 18690413
-
Activation of HuR downstream of p38 MAPK promotes cardiomyocyte hypertrophy.Cell Signal. 2016 Nov;28(11):1735-41. doi: 10.1016/j.cellsig.2016.08.005. Epub 2016 Aug 10. Cell Signal. 2016. PMID: 27521603 Free PMC article.
-
Polydatin attenuates cardiac hypertrophy through modulation of cardiac Ca2+ handling and calcineurin-NFAT signaling pathway.Am J Physiol Heart Circ Physiol. 2014 Sep 1;307(5):H792-802. doi: 10.1152/ajpheart.00017.2014. Epub 2014 Jul 11. Am J Physiol Heart Circ Physiol. 2014. PMID: 25015961
-
Pharmacological Protein Kinase C Modulators Reveal a Pro-hypertrophic Role for Novel Protein Kinase C Isoforms in Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes.Front Pharmacol. 2021 Jan 20;11:553852. doi: 10.3389/fphar.2020.553852. eCollection 2020. Front Pharmacol. 2021. PMID: 33584253 Free PMC article.
References
-
- Olson E.N. A decade of discoveries in cardiac biology. Nat. Med. 2004;10:467–474. - PubMed
-
- Tham Y.K., Bernardo B.C., Ooi J.Y. Pathophysiology of cardiac hypertrophy and heart failure: signaling pathways and novel therapeutic targets. Arch. Toxicol. 2015;89:1401–1438. - PubMed
-
- Loyer X., Heymes C., Samuel J.L. Constitutive nitric oxide synthases in the heart from hypertrophy to failure. Clin. Exp. Pharmacol. Physiol. 2008;35:483–488. - PubMed
-
- Bendall J.K., Damy T., Ratajczak P. Role of myocardial neuronal nitric oxide synthase-derived nitric oxide in beta-adrenergic hyporesponsiveness after myocardial infarction-induced heart failure in rat. Circulation. 2004;110:2368–2375. - PubMed
LinkOut - more resources
Full Text Sources

