The Long Noncoding RNA NEAT1 Targets miR-34a-5p and Drives Nasopharyngeal Carcinoma Progression via Wnt/β-Catenin Signaling
- PMID: 30900419
- PMCID: PMC6433575
- DOI: 10.3349/ymj.2019.60.4.336
The Long Noncoding RNA NEAT1 Targets miR-34a-5p and Drives Nasopharyngeal Carcinoma Progression via Wnt/β-Catenin Signaling
Abstract
Purpose: Long noncoding RNA nuclear paraspeckle assembly transcript 1 (NEAT1) has been deemed an oncogene in many human cancers. However, the underlying mechanism of NEAT1 in nasopharyngeal carcinoma (NPC) progression remains largely unclear.
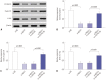
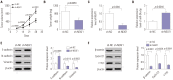
Materials and methods: Quantitative real-time PCR assay was performed to assess the expression of NEAT1 and miR-34a-5p in NPC tissues and cells. Western blot analysis was used to observe cell epithelial to mesenchymal transition (EMT) and the activation of Wnt/β-catenin signaling in 5-8F cells. MiRNA directly interacting with NEAT1 were verified by dual-luciferase reporter assay and RNA immunoprecipitation. Cell proliferation ability was determined by CCK-8 assay, and cell migration and invasion capacities were assessed by transwell assays. An animal model was used to investigate the regulatory effect of NEAT1 on tumor growth in vivo.
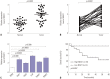
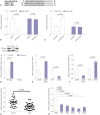
Results: Our data revealed that NEAT1 is upregulated, while miR-34a-5p is downregulated in NPC tissues and cell lines. NEAT1 knockdown repressed tumor growth in vitro and in vivo. Additionally, we discovered that NEAT1 directly binds to miR-34a-5p and suppresses miR-34a-5p expression. Moreover, NEAT1 knockdown exerted suppression effects on cell proliferation, migration, invasion, and EMT by miR-34a-5p. NEAT1 knockdown blocked Wnt/β-catenin signaling via miR-34a-5p.
Conclusion: Our study demonstrated that NEAT1 targets miR-34a-5p at least partly to drive NPC progression by regulating Wnt/β-catenin signaling, suggesting a potential therapeutic target for NPC.
Keywords: Nasopharyngeal carcinoma; Wnt/β-catenin signaling pathway; lncRNA NEAT1; miR-34a-5p.
© Copyright: Yonsei University College of Medicine 2019.
Conflict of interest statement
The authors have no potential conflicts of interest to disclose.
Figures

References
-
- Lee VH, Lam KO, Chang AT, Lam TC, Chiang CL, So TH, et al. Management of nasopharyngeal carcinoma: is adjuvant therapy needed? J Oncol Pract. 2018;14:594–602. - PubMed
-
- Yip TT, Ngan RK, Fong AH, Law SC. Application of circulating plasma/serum EBV DNA in the clinical management of nasopharyngeal carcinoma. Oral Oncol. 2014;50:527–538. - PubMed
-
- Wu F, Wang R, Lu H, Wei B, Feng G, Li G, et al. Concurrent chemoradiotherapy in locoregionally advanced nasopharyngeal carcinoma: treatment outcomes of a prospective, multicentric clinical study. Radiother Oncol. 2014;112:106–111. - PubMed
-
- Lai SZ, Li WF, Chen L, Luo W, Chen YY, Liu LZ, et al. How does intensity-modulated radiotherapy versus conventional two-dimensional radiotherapy influence the treatment results in nasopharyngeal carcinoma patients? Int J Radiat Oncol Biol Phys. 2011;80:661–668. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

