Cortico-hippocampal network connections support the multidimensional quality of episodic memory
- PMID: 30900990
- PMCID: PMC6450667
- DOI: 10.7554/eLife.45591
Cortico-hippocampal network connections support the multidimensional quality of episodic memory
Abstract
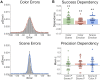
Episodic memories reflect a bound representation of multimodal features that can be reinstated with varying precision. Yet little is known about how brain networks involved in memory, including the hippocampus and posterior-medial (PM) and anterior-temporal (AT) systems, interact to support the quality and content of recollection. Participants learned color, spatial, and emotion associations of objects, later reconstructing the visual features using a continuous color spectrum and 360-degree panorama scenes. Behaviorally, dependencies in memory were observed for the gist but not precision of event associations. Supporting this integration, hippocampus, AT, and PM regions showed increased connectivity and reduced modularity during retrieval compared to encoding. These inter-network connections tracked a multidimensional, objective measure of memory quality. Moreover, distinct patterns of connectivity tracked item color and spatial memory precision. These findings demonstrate how hippocampal-cortical connections reconfigure during episodic retrieval, and how such dynamic interactions might flexibly support the multidimensional quality of remembered events.
Keywords: cortical networks; episodic memory; functional connectivity; hippocampus; human; memory precision; neuroscience; spatial memory.
© 2019, Cooper and Ritchey.
Conflict of interest statement
RC, MR No competing interests declared
Figures




References
-
- Bradley MM, Lang PJ. The International Affective Digitized Sounds. 2nd Edition. Gainesville: University of Florida; 2007. Affective ratings of sounds and instruction manual. Technical report B-3.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

