Role of T-cell activation in salt-sensitive hypertension
- PMID: 30901277
- PMCID: PMC6620682
- DOI: 10.1152/ajpheart.00096.2019
Role of T-cell activation in salt-sensitive hypertension
Abstract
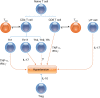
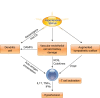
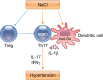
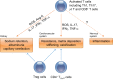
The contributions of T lymphocytes to the pathogenesis of salt-sensitive hypertension has been well established. Under hypertensive stimuli, naive T cells develop into different subsets, including Th1, Th2, Th17, Treg, and cytotoxic CD8+ T cells, depending on the surrounding microenviroment in organs. Distinct subsets of T cells may play totally different roles in tissue damage and hypertension. The underlying mechanisms by which hypertensive stimuli activate naive T cells involve many events and different organs, such as neoantigen presentation by dendritic cells, high salt concentration, and the milieu of oxidative stress in the kidney and vasculature. Infiltrating and activated T subsets in injured organs, in turn, exert considerable impacts on tissue dysfunction, including sodium retention in the kidney, vascular stiffness, and remodeling in the vasculature. Therefore, a thorough knowledge of T-cell actions in hypertension may provide novel insights into the development of new therapeutic strategies for patients with hypertension.
Keywords: T cell; end-organ damage; hypertension; salt.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures
References
-
- Banek CT, Knuepfer MM, Foss JD, Fiege JK, Asirvatham-Jeyaraj N, Van Helden D, Shimizu Y, Osborn JW. Resting afferent renal nerve discharge and renal inflammation: elucidating the role of afferent and efferent renal nerves in deoxycorticosterone acetate salt hypertension. Hypertension 68: 1415–1423, 2016. doi:10.1161/HYPERTENSIONAHA.116.07850. - DOI - PMC - PubMed
-
- Barbaro NR, Foss JD, Kryshtal DO, Tsyba N, Kumaresan S, Xiao L, Mernaugh RL, Itani HA, Loperena R, Chen W, Dikalov S, Titze JM, Knollmann BC, Harrison DG, Kirabo A. Dendritic cell amiloride-sensitive channels mediate sodium-induced inflammation and hypertension. Cell Reports 21: 1009–1020, 2017. doi:10.1016/j.celrep.2017.10.002. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

