Randomized Phase III Trial of Ibrutinib and Rituximab Plus Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone in Non-Germinal Center B-Cell Diffuse Large B-Cell Lymphoma
- PMID: 30901302
- PMCID: PMC6553835
- DOI: 10.1200/JCO.18.02403
Randomized Phase III Trial of Ibrutinib and Rituximab Plus Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone in Non-Germinal Center B-Cell Diffuse Large B-Cell Lymphoma
Abstract
Purpose: Ibrutinib has shown activity in non-germinal center B-cell diffuse large B-cell lymphoma (DLBCL). This double-blind phase III study evaluated ibrutinib and rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) in untreated non-germinal center B-cell DLBCL.
Patients and methods: Patients were randomly assigned at a one-to-one ratio to ibrutinib (560 mg per day orally) plus R-CHOP or placebo plus R-CHOP. The primary end point was event-free survival (EFS) in the intent-to-treat (ITT) population and the activated B-cell (ABC) DLBCL subgroup. Secondary end points included progression-free survival (PFS), overall survival (OS), and safety.
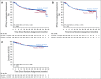
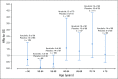
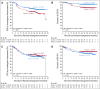
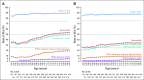
Results: A total of 838 patients were randomly assigned to ibrutinib plus R-CHOP (n = 419) or placebo plus R-CHOP (n = 419). Median age was 62.0 years; 75.9% of evaluable patients had ABC subtype disease, and baseline characteristics were balanced. Ibrutinib plus R-CHOP did not improve EFS in the ITT (hazard ratio [HR], 0.934) or ABC (HR, 0.949) population. A preplanned analysis showed a significant interaction between treatment and age. In patients age younger than 60 years, ibrutinib plus R-CHOP improved EFS (HR, 0.579), PFS (HR, 0.556), and OS (HR, 0.330) and slightly increased serious adverse events (35.7% v 28.6%), but the proportion of patients receiving at least six cycles of R-CHOP was similar between treatment arms (92.9% v 93.0%). In patients age 60 years or older, ibrutinib plus R-CHOP worsened EFS, PFS, and OS, increased serious adverse events (63.4% v 38.2%), and decreased the proportion of patients receiving at least six cycles of R-CHOP (73.7% v 88.8%).
Conclusion: The study did not meet its primary end point in the ITT or ABC population. However, in patients age younger than 60 years, ibrutinib plus R-CHOP improved EFS, PFS, and OS with manageable safety. In patients age 60 years or older, ibrutinib plus R-CHOP was associated with increased toxicity, leading to compromised R-CHOP administration and worse outcomes. Further investigation is warranted.
Trial registration: ClinicalTrials.gov NCT01855750.
Figures



Comment in
-
De-Cell-eration in Therapy for Diffuse Large B-Cell Lymphoma.J Clin Oncol. 2019 May 20;37(15):1267-1269. doi: 10.1200/JCO.19.00445. Epub 2019 Apr 2. J Clin Oncol. 2019. PMID: 30945958 No abstract available.
-
Reply to M. Skelin et al.J Clin Oncol. 2019 Oct 1;37(28):2584-2585. doi: 10.1200/JCO.19.01603. Epub 2019 Aug 7. J Clin Oncol. 2019. PMID: 31390273 No abstract available.
-
Above What Age Should Ibrutinib Not Be Given With R-CHOP to Patients With Non-GBC DLBCL?J Clin Oncol. 2019 Oct 1;37(28):2583-2584. doi: 10.1200/JCO.19.01048. Epub 2019 Aug 7. J Clin Oncol. 2019. PMID: 31390275 No abstract available.
References
-
- Stewart BW, Wild CP: World Cancer Report 2014. Lyon, France, IARC Press, 2014.
-
- Alizadeh AA, Eisen MB, Davis RE, et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature. 2000;403:503–511. - PubMed
-
- Hans CP, Weisenburger DD, Greiner TC, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004;103:275–282. - PubMed
-
- Tilly H, Gomes da Silva M, Vitolo U, et al: Diffuse large B-cell lymphoma (DLBCL): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 26:v116-v125, 2015 (suppl 5) - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

