The Genetic Basis of Metabolic Disease
- PMID: 30901536
- PMCID: PMC6432945
- DOI: 10.1016/j.cell.2019.02.024
The Genetic Basis of Metabolic Disease
Abstract
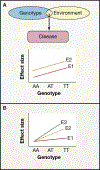
Recent developments in genetics and genomics are providing a detailed and systematic characterization of the genetic underpinnings of common metabolic diseases and traits, highlighting the inherent complexity within systems for homeostatic control and the many ways in which that control can fail. The genetic architecture underlying these common metabolic phenotypes is complex, with each trait influenced by hundreds of loci spanning a range of allele frequencies and effect sizes. Here, we review the growing appreciation of this complexity and how this has fostered the implementation of genome-scale approaches that deliver robust mechanistic inference and unveil new strategies for translational exploitation.
Copyright © 2019 Elsevier Inc. All rights reserved.
Figures



References
-
- Altshuler D, Hirschhorn JN, Klannemark M, Lindgren CM, Vohl MC, Nemesh J, Lane CR, Schaffner SF, Bolk S, Brewer C, et al. (2000). “The common PPARgamma Pro12Ala polymorphism is associated with decreased risk of type 2 diabetes.” Nat Genet 26(1): 76–80. - PubMed
-
- Ameur A, Enroth S, Johansson A, Zaboli G, Igl W, Johansson AC, Rivas MA, Daly MJ, Schmitz G, Hicks AA, et al. (2012). “Genetic adaptation of fatty-acid metabolism: a human-specific haplotype increasing the biosynthesis of long-chain omega-3 and omega-6 fatty acids.” Am J Hum Genet 90(5): 809–820. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

