AKTivation mechanisms
- PMID: 30901610
- PMCID: PMC6752985
- DOI: 10.1016/j.sbi.2019.02.004
AKTivation mechanisms
Abstract
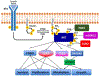
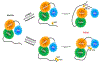
Akt1-3 (Akt) are a subset of the AGC protein Ser/Thr kinase family and play important roles in cell growth, metabolic regulation, cancer, and other diseases. We describe some of the roles of Akt in cell signaling and the biochemical and structural mechanisms of the regulation of Akt catalysis by the phospholipid PIP3 and by phosphorylation. Recent findings highlight a diverse set of strategies to control Akt catalytic activity to ensure its normal biological functions.
Copyright © 2019 Elsevier Ltd. All rights reserved.
Figures
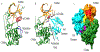
References
-
- Parang K, Till JH, Ablooglu AJ, Kohanski RA, Hubbard SR, Cole PA: Mechanism-based design of a protein kinase inhibitor. Nat Struct Biol 2001, 8:37–41. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

