Long-Term Effects of Initiating Continuous Subcutaneous Insulin Infusion (CSII) and Continuous Glucose Monitoring (CGM) in People with Type 1 Diabetes and Unsatisfactory Diabetes Control
- PMID: 30901914
- PMCID: PMC6463068
- DOI: 10.3390/jcm8030394
Long-Term Effects of Initiating Continuous Subcutaneous Insulin Infusion (CSII) and Continuous Glucose Monitoring (CGM) in People with Type 1 Diabetes and Unsatisfactory Diabetes Control
Abstract
Background: We aimed to assess the long-term effects of the introduction of continuous subcutaneous insulin infusion (CSII) and continuous glucose monitoring (CGM) in people with type 1 diabetes (T1D).
Methods: A prospective single-centre cohort study including participants with T1D and HbA1c > 7.5%. After completing a course in flexible intensified insulin treatment (FIT), participants were offered treatment change to CSII/CGM. FIT participants with HbA1c ≤ 7.5% who remained on multiple daily injections (MDI) and without CGM were monitored as a separate cohort to compare the cumulative incidence of diabetic complications.
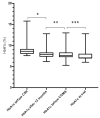
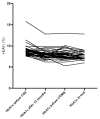
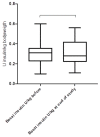
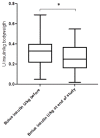
Results: The study cohort included 41 participants with T1D (21 male/20 female). The mean age (±SD) at inclusion was 24.2 ± 10.9 years, the mean follow-up was 8.9 ± 2.8 years, and the mean diabetes duration at the end of the study was 15.9 ± 10.1 years. The mean HbA1c level before the introduction of CSII was 8.8 ± 1.3% (73 ± 8 mmol/mol), and decreased significantly thereafter to 8.0 ± 1.1% (63 ± 7 mmol/mol) (p = 0.0001), and further to 7.6 ± 1.1% (59 ± 11 mmol/mol) after the initiation of CGM (p = 0.051). In the MDI group the HbA1c levels did not change significantly during a mean follow-up of 6.8 ± 3.2 years. The frequency of severe hypoglycaemia after the introduction of CSII/CGM declined significantly (from 9.7 to 2.2 per 100 patient-years, p = 0.03), and the cumulative incidence of newly diagnosed diabetic microvascular complications were comparable between the study group and the observational cohort.
Conclusion: In people with T1D and unsatisfactory diabetes control the introduction of CSII and CGM results in a substantial and long-term improvement.
Keywords: Type 1 diabetes; continuous glucose monitoring; continuous subcutaneous insulin infusion; functional insulin therapy; insulin pump.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Writing Group for the DCCT/EDIC Research Group. Orchard T.J., Nathan D.M., Zinman B., Cleary P., Brillon D., Backlund J.Y., Lachin J.M. Association Between 7 Years of Intensive Treatment of Type 1 Diabetes and Long-term Mortality. JAMA. 2015;313:45–53. doi: 10.1001/jama.2014.16107. - DOI - PMC - PubMed
-
- Albrecht D., Puder J., Keller U., Zulewski H. Potential of education-based insulin therapy for achievement of good metabolic control: a real-life experience: Potential of education-based insulin therapy for Type 1 diabetes. Diabet. Med. 2011;28:539–542. doi: 10.1111/j.1464-5491.2011.03260.x. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

