The Significance of Lactoperoxidase System in Oral Health: Application and Efficacy in Oral Hygiene Products
- PMID: 30901933
- PMCID: PMC6472183
- DOI: 10.3390/ijms20061443
The Significance of Lactoperoxidase System in Oral Health: Application and Efficacy in Oral Hygiene Products
Abstract
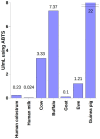
Lactoperoxidase (LPO) present in saliva are an important element of the nonspecific immune response involved in maintaining oral health. The main role of this enzyme is to oxidize salivary thiocyanate ions (SCN-) in the presence of hydrogen peroxide (H₂O₂) to products that exhibit antimicrobial activity. LPO derived from bovine milk has found an application in food, cosmetics, and medical industries due to its structural and functional similarity to the human enzyme. Oral hygiene products enriched with the LPO system constitute an alternative to the classic fluoride caries prophylaxis. This review describes the physiological role of human salivary lactoperoxidase and compares the results of clinical trials and in vitro studies of LPO alone and complex dentifrices enriched with bovine LPO. The role of reactivators and inhibitors of LPO is discussed together with the possibility of using nanoparticles to increase the stabilization and activity of this enzyme.
Keywords: caries prophylaxis; dentifrice; lactoperoxidase; periodontitis prophylaxis; saliva.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Gudipaneni R.K., Kumar R.V., Jesudass G., Peddengatagari S., Duddu Y. Short term comparative evaluation of antimicrobial efficacy of tooth paste containing lactoferrin, lysozyme, lactoperoxidase in children with severe early childhood caries: A clinical study. J. Clin. Diagn. Res. 2014;8:ZC18–ZC20. doi: 10.7860/JCDR/2014/8161.4232. - DOI - PMC - PubMed
-
- Shimizu E., Kobayashi T., Wakabayashi H., Yamauchi K., Iwatsuki K., Yoshie H. Effects of Orally Administered Lactoferrin and Lactoperoxidase-Containing Tablets on Clinical and Bacteriological Profiles in Chronic Periodontitis Patients. Int. J. Dent. 2011;2011:1–9. doi: 10.1155/2011/405139. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

