Function, Architecture, and Biogenesis of Reovirus Replication Neoorganelles
- PMID: 30901959
- PMCID: PMC6466366
- DOI: 10.3390/v11030288
Function, Architecture, and Biogenesis of Reovirus Replication Neoorganelles
Abstract
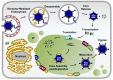
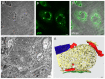
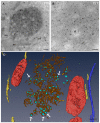
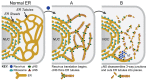
Most viruses that replicate in the cytoplasm of host cells form neoorganelles that serve as sites of viral genome replication and particle assembly. These highly specialized structures concentrate viral proteins and nucleic acids, prevent the activation of cell-intrinsic defenses, and coordinate the release of progeny particles. Reoviruses are common pathogens of mammals that have been linked to celiac disease and show promise for oncolytic applications. These viruses form nonenveloped, double-shelled virions that contain ten segments of double-stranded RNA. Replication organelles in reovirus-infected cells are nucleated by viral nonstructural proteins µNS and σNS. Both proteins partition the endoplasmic reticulum to form the matrix of these structures. The resultant membranous webs likely serve to anchor viral RNA⁻protein complexes for the replication of the reovirus genome and the assembly of progeny virions. Ongoing studies of reovirus replication organelles will advance our knowledge about the strategies used by viruses to commandeer host biosynthetic pathways and may expose new targets for therapeutic intervention against diverse families of pathogenic viruses.
Keywords: Reovirus; bluetongue virus; double-stranded RNA; endoplasmic reticulum; rotavirus; viral factories; viral inclusions; viral nonstructural proteins; viral replication organelles.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

