Roles and regulation of SOX transcription factors in skeletogenesis
- PMID: 30902252
- PMCID: PMC6955022
- DOI: 10.1016/bs.ctdb.2019.01.007
Roles and regulation of SOX transcription factors in skeletogenesis
Abstract
SOX transcription factors participate in the specification, differentiation and activities of many cell types in development and beyond. The 20 mammalian family members are distributed into eight groups based on sequence identity, and while co-expressed same-group proteins often have redundant functions, different-group proteins typically have distinct functions. More than a handful of SOX proteins have pivotal roles in skeletogenesis. Heterozygous mutations in their genes cause human diseases, in which skeletal dysmorphism is a major feature, such as campomelic dysplasia (SOX9), or a minor feature, such as LAMSHF syndrome (SOX5) and Coffin-Siris-like syndromes (SOX4 and SOX11). Loss- and gain-of-function experiments in animal models have revealed that SOX4 and SOX11 (SOXC group) promote skeletal progenitor survival and control skeleton patterning and growth; SOX8 (SOXE group) delays the differentiation of osteoblast progenitors; SOX9 (SOXE group) is essential for chondrocyte fate maintenance and differentiation, and works in cooperation with SOX5 and SOX6 (SOXD group) and other types of transcription factors. These and other SOX proteins have also been proposed, mainly through in vitro experiments, to have key roles in other aspects of skeletogenesis, such as SOX2 in osteoblast stem cell self-renewal. We here review current knowledge of well-established and proposed skeletogenic roles of SOX proteins, their transcriptional and non-transcriptional actions, and their modes of regulation at the gene, RNA and protein levels. We also discuss gaps in knowledge and directions for future research to further decipher mechanisms underlying skeletogenesis in health and diseases and identify treatment options for skeletal malformation and degeneration diseases.
Keywords: Cell differentiation; Cell specification; Chondrocyte; Gene regulation; Osteoblast; SOX; Skeletogenesis; Transcription factor; Transcriptional regulation.
© 2019 Elsevier Inc. All rights reserved.
Figures
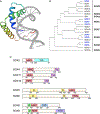
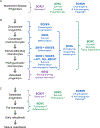
References
-
- Amarilio R, Viukov SV, Sharir A, Eshkar-Oren I, Johnson RS, & Zelzer E (2007). HIF1alpha regulation of Sox9 is necessary to maintain differentiation of hypoxic prechondrogenic cells during early skeletogenesis. Development, 134, 3917–3928. - PubMed
-
- Bagheri-Fam S, Barrionuevo F, Dohrmann U, Gunther T, Schule R, Kemler R, et al. (2006). Long-range upstream and downstream enhancers control distinct subsets of the complex spatiotemporal Sox9 expression pattern. Developmental Biology, 291, 382–397. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

