NLRP12 Regulates Anti-viral RIG-I Activation via Interaction with TRIM25
- PMID: 30902577
- PMCID: PMC6459718
- DOI: 10.1016/j.chom.2019.02.013
NLRP12 Regulates Anti-viral RIG-I Activation via Interaction with TRIM25
Abstract
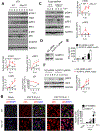
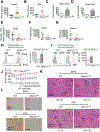
Establishing the balance between positive and negative innate immune mechanisms is crucial for maintaining homeostasis. Here we uncover the regulatory crosstalk between two previously unlinked innate immune receptor families: RIG-I, an anti-viral cytosolic receptor activated type I interferon production, and NLR (nucleotide-binding domain, leucine repeat domain-containing protein). We show that NLRP12 dampens RIG-I-mediated immune signaling against RNA viruses by controlling RIG-I's association with its adaptor MAVS. The nucleotide-binding domain of NLRP12 interacts with the ubiquitin ligase TRIM25 to prevent TRIM25-mediated, Lys63-linked ubiquitination and activation of RIG-I. NLRP12 also enhances RNF125-mediated, Lys48-linked degradative ubiquitination of RIG-I. Vesicular stomatitis virus (VSV) infection downregulates NLRP12 expression to allow RIG-I activation. Myeloid-cell-specific Nlrp12-deficient mice display a heightened interferon and TNF response and are more resistant to VSV infection. These results indicate that NLRP12 functions as a checkpoint for anti-viral RIG-I activation.
Copyright © 2019 Elsevier Inc. All rights reserved.
Conflict of interest statement
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
Figures





Similar articles
-
Human Respiratory Syncytial Virus NS 1 Targets TRIM25 to Suppress RIG-I Ubiquitination and Subsequent RIG-I-Mediated Antiviral Signaling.Viruses. 2018 Dec 14;10(12):716. doi: 10.3390/v10120716. Viruses. 2018. PMID: 30558248 Free PMC article.
-
A RIG-I-like receptor directs antiviral responses to a bunyavirus and is antagonized by virus-induced blockade of TRIM25-mediated ubiquitination.J Biol Chem. 2020 Jul 10;295(28):9691-9711. doi: 10.1074/jbc.RA120.013973. Epub 2020 May 29. J Biol Chem. 2020. PMID: 32471869 Free PMC article.
-
A distinct role of Riplet-mediated K63-Linked polyubiquitination of the RIG-I repressor domain in human antiviral innate immune responses.PLoS Pathog. 2013;9(8):e1003533. doi: 10.1371/journal.ppat.1003533. Epub 2013 Aug 8. PLoS Pathog. 2013. PMID: 23950712 Free PMC article.
-
Recent Advances and Contradictions in the Study of the Individual Roles of Ubiquitin Ligases That Regulate RIG-I-Like Receptor-Mediated Antiviral Innate Immune Responses.Front Immunol. 2020 Jun 24;11:1296. doi: 10.3389/fimmu.2020.01296. eCollection 2020. Front Immunol. 2020. PMID: 32670286 Free PMC article. Review.
-
[Innate immune response to RNA virus infection].Uirusu. 2011 Dec;61(2):153-61. doi: 10.2222/jsv.61.153. Uirusu. 2011. PMID: 22916562 Review. Japanese.
Cited by
-
Quantitative trait loci and genes associated with salmonid alphavirus load in Atlantic salmon: implications for pancreas disease resistance and tolerance.Sci Rep. 2020 Jun 25;10(1):10393. doi: 10.1038/s41598-020-67405-8. Sci Rep. 2020. PMID: 32587341 Free PMC article.
-
The role of pyroptosis in cancer: key components and therapeutic potential.Cell Commun Signal. 2024 Nov 15;22(1):548. doi: 10.1186/s12964-024-01932-z. Cell Commun Signal. 2024. PMID: 39548573 Free PMC article. Review.
-
Innate Immunity Never "NODs" Off: NLRs Regulate the Host Anti-Viral Immune Response.Immunol Rev. 2025 Mar;330(1):e13429. doi: 10.1111/imr.13429. Immunol Rev. 2025. PMID: 39878363 Free PMC article. Review.
-
The Roles of TRIMs in Antiviral Innate Immune Signaling.Front Cell Infect Microbiol. 2021 Mar 15;11:628275. doi: 10.3389/fcimb.2021.628275. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 33791238 Free PMC article. Review.
-
Inflammasome activation and regulation: toward a better understanding of complex mechanisms.Cell Discov. 2020 Jun 9;6:36. doi: 10.1038/s41421-020-0167-x. eCollection 2020. Cell Discov. 2020. PMID: 32550001 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

