Multiple Immune-Suppressive Mechanisms in Fibrolamellar Carcinoma
- PMID: 30902819
- PMCID: PMC9474294
- DOI: 10.1158/2326-6066.CIR-18-0499
Multiple Immune-Suppressive Mechanisms in Fibrolamellar Carcinoma
Abstract
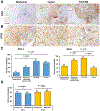
Fibrolamellar carcinoma (FLC) is a rare type of liver cancer that affects adolescents and young adults. The most effective treatment for FLC is surgical resection, but no standardized systemic therapy exists for patients with recurrent or unresectable FLC. As a first step to understand the immune microenvironment of FLC, we investigated targetable immune-checkpoint pathways, PD-1, PD-L1, B7-H3, IDO-1, and LAG3, in relation to CD8+ cytotoxic T-lymphocyte density. Thirty-two FLC tumor specimens were analyzed using IHC staining for PD-L1, CD8, PD-1, IDO, LAG3, and B7-H3. Sixty-three percent of FLC cases demonstrated membranous PD-L1 expression on tumor cells, and almost 70% of cases demonstrated PD-L1+ tumor-infiltrating lymphocytes and tumor-associated macrophages (TIL/TAM). Myeloid-derived cells appeared to be a major component of PD-L1+ tumor-infiltrating immune cells. Forty percent of the cases showed B7-H3 expression in the tumor zone, with 91% cases showing B7-H3 expression in TILs and TAMs. IDO and PD-1 expression was highest in the tumor interface zone. B7-H3 or IDO expression on tumor cells significantly correlated with higher CD8+ T-cell density. In conclusion, a high proportion of FLC cases showed robust expression of PD-1, PD-L1, B7-H3, and IDO in an adaptive immune-resistance pattern. Our findings provide further basis for targeting these different immune-checkpoint axes in FLC.
©2019 American Association for Cancer Research.
Figures





References
-
- El-Serag HB, Davila JA. Is fibrolamellar carcinoma different from hepatocellular carcinoma? A US population-based study. Hepatology 2004;39: 798–803. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

