Extrinsic and intrinsic apoptosis activate pannexin-1 to drive NLRP3 inflammasome assembly
- PMID: 30902848
- PMCID: PMC6517827
- DOI: 10.15252/embj.2019101638
Extrinsic and intrinsic apoptosis activate pannexin-1 to drive NLRP3 inflammasome assembly
Abstract
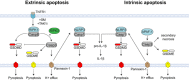
Pyroptosis is a form of lytic inflammatory cell death driven by inflammatory caspase-1, caspase-4, caspase-5 and caspase-11. These caspases cleave and activate the pore-forming protein gasdermin D (GSDMD) to induce membrane damage. By contrast, apoptosis is driven by apoptotic caspase-8 or caspase-9 and has traditionally been classified as an immunologically silent form of cell death. Emerging evidence suggests that therapeutics designed for cancer chemotherapy or inflammatory disorders such as SMAC mimetics, TAK1 inhibitors and BH3 mimetics promote caspase-8 or caspase-9-dependent inflammatory cell death and NLRP3 inflammasome activation. However, the mechanism by which caspase-8 or caspase-9 triggers cell lysis and NLRP3 activation is still undefined. Here, we demonstrate that during extrinsic apoptosis, caspase-1 and caspase-8 cleave GSDMD to promote lytic cell death. By engineering a novel Gsdmd D88A knock-in mouse, we further demonstrate that this proinflammatory function of caspase-8 is counteracted by caspase-3-dependent cleavage and inactivation of GSDMD at aspartate 88, and is essential to suppress GSDMD-dependent cell lysis during caspase-8-dependent apoptosis. Lastly, we provide evidence that channel-forming glycoprotein pannexin-1, but not GSDMD or GSDME promotes NLRP3 inflammasome activation during caspase-8 or caspase-9-dependent apoptosis.
Keywords: NLRP3; apoptosis; gasdermin; pannexin‐1; pyroptosis.
© 2019 The Authors.
Conflict of interest statement
A.B and C.J.F are employees of Novartis, Inc.
Figures
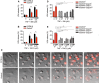
- A–E
Primary (A, B) or immortalized BMDMs (D, E) were stimulated with recombinant murine TNF (100 ng/ml) in combination with (A, D) SM or (B, E) TAK1i for 6 or 4 h, respectively. (C) Time‐lapse confocal images (hour:min) of BMDMs stimulated with recombinant murine TNF (100 ng/ml) and SM (250 nM) stained with propidium iodide (red) for 6 h. Black arrowheads indicate membrane ballooning, while white arrowheads indicate apoptotic bodies.

- A, B
BMDMs were stimulated with TNF (100 ng/ml) in combination with TAK1i (125 nM) for the indicated time points. (A) LDH release and (B) mixed supernatant and cell extracts were analysed.
- C
Representation of known caspase cleavage site and molecular weight of corresponding cleavage fragment in mouse GSDMD.
- D
BMDMs were costimulated with TNF (100 ng/ml) and TAK1i (125 nM) for 4 h in the presence or absence of KCl (50 mM). Where indicated, cells were pre‐incubated with MCC950 (10 μM) 20–30 min prior to TNF/TAK1i stimulation.
- E–G
BMDMs were costimulated with TNF (100 ng/ml) and SM (E) (250 nM; 6 h), mixed supernatant and cell extracts were analysed by immunoblot, or (F) LDH release in the cell culture supernatant was quantified at the indicated time points.
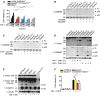
- A
BMDMs were costimulated with TNF (100 ng/ml) and SM (500 nM) for the indicated time points, and LDH release was quantified.
- B, C
Immortalized BMDMs were (B) costimulated with TNF (100 ng/ml) and SM (500 nM) for 6 h or (C) primed with ultrapure E. coli K12 LPS (100 ng/ml) for 3 h prior to stimulation with the SMAC‐mimetic LCL161 (1 μM) for a further 16 h. Mixed supernatant and extracts were analysed by immunoblot.
- D
BMDM were costimulated with ultrapure E. coli K12 LPS (100 ng/ml) or TNF (100 ng/ml) and TAK1i (125 nM) for 2 or 4 h, and mixed supernatant and extracts were analysed by immunoblot.
- E
BMDMs were costimulated with TNF (100 ng/ml) and SM (500 nM) for 6 h, and mixed supernatant and extracts were analysed by immunoblot.
- F
BMDMs were costimulated with TNF (100 ng/ml) and TAK1i (125 nM), and LDH release was quantified at 4 h.

BMDMs were stimulated with TNF (100 ng/ml) and SM (500 nM) for 6 h, and mixed supernatant and extracts were analysed by immunoblot, representative of three independent experiments.
Caspase‐1 expression in HEK293T versus HeLa cells.
Amino acid sequence of human gasdermin D. The fluorescence lifetime substrate Ac‐Cys(Pt14)‐FLTD^GVPY‐NH2 was designed around D276 as highlighted (red); ^ indicates the Casp1/8 cleavage site.
The kinetic constants of the proteolysis of the FLT‐substrate Ac‐Cys(Pt14)‐FLTD^GVPY‐NH2 by Casp1/8 were determined from the time courses of product formation under initial velocity conditions. The K M value was obtained from measurements conducted at constant enzyme concentration (Casp1 = 30 nM; Casp8 = 833 nM) and different substrate concentrations as indicated.
Comparison of kinetic constants determined for caspase‐1/8 cleavage of (Pt14)‐LETD^Y‐NH2 and Ac‐Cys(Pt14)‐FLTD^GVPY‐NH2.

- A
HEK293T cells were transfected with doxycycline‐inducible DmrB‐caspase‐8 and the indicated GSDMD constructs. Cells were stimulated with doxycycline (10 μg/ml) for 18 h to induce DmrB‐caspase‐8 expression and exposed to B/B homodimerizer (12.5 nM) for another 2 h to activate caspase‐8. Mixed supernatant and extracts were analysed by immunoblot.
- B, C
Immortalized Gsdmd −/− BMDM expressing GSDMDWT and GSDMDD88A were (B) costimulated with TNF (100 ng/ml) and SM for 6 h or (C) primed for 3 h with ultrapure E. coli K12 LPS (100 ng/ml) and stimulated with LCL161 (1 μM) for 24 h, and LDH release was quantified.
- D, E
BMDMs were costimulated with TNF (100 ng/ml) and TAK1i for 4 h, (D) mixed supernatant and extracts were analysed by immunoblot, or (E) LDH release was quantified at the indicated time points.

- A, B
BMDMs were costimulated with TNF (100 ng/ml) and SM (500 nM), (A) LDH release was quantified at the indicated time points, or (B) mixed supernatant and extracts were analysed at 5 h. (A) Data are means ± SEM of pooled data from three independent experiments. Statistical analyses were performed using a two‐way ANOVA Data were considered significant when **P < 0.01 or ****P < 0.0001. (B) Immunoblots are representative of three independent experiments.

- A–E
BMDMs were costimulated with TNF (100 ng/ml) and TAK1i (125 nM) for 4 h, (A, B) LDH release was quantified, or (C, D, E) mixed supernatant and extracts were analysed by immunoblot. Where indicated, cells were treated with the inhibitors Nec‐1s (50 μM), MCC950 (10 μM), GSK'872 (1 μM), probenecid (1 mM) 20–30 min prior to cell stimulation. KCl (50 mM) was added together with TNF and TAK1i. (C) To induce necroptosis, BMDMs were primed for 3 h with ultrapure E. coli K12 LPS (100 ng/ml) and Q‐VD‐OPh (10 μM) was added at the last 20–30 min of priming and stimulated with SM (500 nM) for 4 h. (E) To activate the NLRP3 inflammasome, BMDMs were primed with ultrapure E. coli K12 LPS (100 ng/ml) for 4 h and stimulated with nigericin (10 μM) for 1 h.
- F
BMDMs were primed for 3 h with ultrapure E. coli K12 LPS (100 ng/ml) and stimulated with SM (0.5 μM) for a further 4 h. Probenecid (1 mM) was added 20–30 min prior to cell stimulation, and mixed supernatant and extracts were analysed by immunoblot.
- G, H
BMDMs were stimulated with TNF (100 ng/ml) and SM (0.5 μM) for 6 h, (G) mixed supernatant and extracts were analysed by immunoblot, or (H) LDH release was quantified.

- A–C
BMDMs were stimulated with TNF (100 ng/ml) or E. coli K12 LPS (100 ng/ml) and TAK1i (125 nM) for 4 h, mixed supernatant and extracts were analysed by immunoblot (A), or (B‐C) LDH release was quantified.
- D
Unprimed BMDMs were stimulated with ABT‐737 (500 nM) and S63845 (500 nM) for 6 h, and mixed supernatant and extracts were analysed by immunoblot.
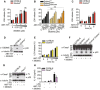
- A, B
BMDMs were stimulated with an increasing dose of S63845 in the presence of ABT‐737 (0.5 μM), and LDH release was quantified at 6 h.
- C–F
BMDMs were stimulated with ABT‐737 (0.5 μM) and S63845 (0.5 μM), LDH release was quantified (C, E), or mixed supernatant and extracts were analysed by immunoblot at 6 h (D, F).
- G, H
BMDMs were primed with ultrapure E. coli K12 LPS (100 ng/ml) for 3 h and further stimulated with ABT‐737 (1 μM) and S63845 (1 μM) for 24 h, mixed supernatant and extracts were analysed by immunoblot (G), and IL‐1β in cell‐free supernatant was quantified by ELISA (H).
Comment in
-
Chopping GSDMD: caspase-8 has joined the team of pyroptosis-mediating caspases.EMBO J. 2019 May 15;38(10):e102065. doi: 10.15252/embj.2019102065. Epub 2019 Apr 15. EMBO J. 2019. PMID: 30988015 Free PMC article.
References
-
- Ahmad K (2001) Drug company sued over research trial in Nigeria. Lancet 358: 815 - PubMed
-
- Broz P, Dixit VM (2016) Inflammasomes: mechanism of assembly, regulation and signalling. Nat Rev Immunol 16: 407–420 - PubMed
-
- Chauhan D, Bartok E, Gaidt MM, Bock FJ, Herrmann J, Seeger JM, Broz P, Beckmann R, Kashkar H, Tait SWG, Muller R, Hornung V (2018) BAX/BAK‐induced apoptosis results in caspase‐8‐dependent IL‐1beta maturation in macrophages. Cell Rep 25: 2354–2368.e5 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

