Astrocytic Epoxyeicosatrienoic Acid Signaling in the Medial Prefrontal Cortex Modulates Depressive-like Behaviors
- PMID: 30902874
- PMCID: PMC6554635
- DOI: 10.1523/JNEUROSCI.3069-18.2019
Astrocytic Epoxyeicosatrienoic Acid Signaling in the Medial Prefrontal Cortex Modulates Depressive-like Behaviors
Abstract
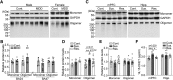
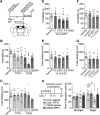
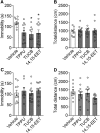
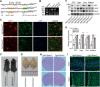
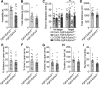
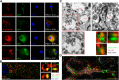
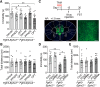
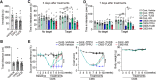
Major depressive disorder is the most common mental illness. Mounting evidence indicates that astrocytes play a crucial role in the pathophysiology of depression; however, the underlying molecular mechanisms remain elusive. Compared with other neuronal cell types, astrocytes are enriched for arachidonic acid metabolism. Herein, we observed brain-region-specific alterations of epoxyeicosatrienoic acid (EET) signaling, which is an arachidonic acid metabolic pathway, in both a mouse model of depression and postmortem samples from patients with depression. The enzymatic activity of soluble epoxide hydrolase (sEH), the key enzyme in EET signaling, was selectively increased in the mPFC of susceptible mice after chronic social defeated stress and was negatively correlated with the social interaction ratio, which is an indicator of depressive-like behavior. The specific deletion of Ephx2 (encode sEH) in adult astrocytes induced resilience to stress, whereas the impaired EET signaling in the mPFC evoked depressive-like behaviors in response to stress. sEH was mainly expressed on lysosomes of astrocytes. Using pharmacological and genetic approaches performed on C57BL/6J background adult male mice, we found that EET signaling modulated astrocytic ATP release in vitro and in vivo Moreover, astrocytic ATP release was required for the antidepressant-like effect of Ephx2 deletion in adult astrocytes. In addition, sEH inhibitors produced rapid antidepressant-like effects in multiple animal models of depression, including chronic social defeated stress and chronic mild stress. Together, our results highlight that EET signaling in astrocytes in the mPFC is essential for behavioral adaptation in response to psychiatric stress.SIGNIFICANCE STATEMENT Astrocytes, the most abundant glial cells of the brain, play a vital role in the pathophysiology of depression. Astrocytes secrete adenosine ATP, which modulates depressive-like behaviors. Notably, astrocytes are enriched for arachidonic acid metabolism. In the present study, we explored the hypothesis that epoxyeicosatrienoic acid signaling, an arachidonic acid metabolic pathway, modulates astrocytic ATP release and the expression of depressive-like behaviors. Our work demonstrated that epoxyeicosatrienoic acid signaling in astrocytes in the mPFC is essential for behavioral homeostatic adaptation in response to stress, and the extent of astrocyte functioning is greater than expected based on earlier reports.
Keywords: ATP; astrocytes; depression; epoxyeicosatrienoic acid signaling; medial prefrontal cortex; soluble epoxide hydrolase.
Copyright © 2019 the authors.
Figures





References
-
- Banasr M, Chowdhury GM, Terwilliger R, Newton SS, Duman RS, Behar KL, Sanacora G (2010) Glial pathology in an animal model of depression: reversal of stress-induced cellular, metabolic and behavioral deficits by the glutamate-modulating drug riluzole. Mol Psychiatry 15:501–511. 10.1038/mp.2008.106 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
