Boundary activated hydrogen evolution reaction on monolayer MoS2
- PMID: 30902982
- PMCID: PMC6430794
- DOI: 10.1038/s41467-019-09269-9
Boundary activated hydrogen evolution reaction on monolayer MoS2
Abstract
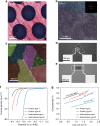
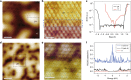
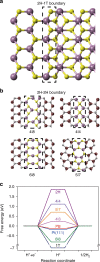
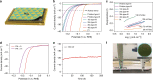
Recently, monolayer molybdenum disulphide (MoS2) has emerged as a promising and non-precious electrocatalyst for hydrogen evolution reaction. However, its performance is largely limited by the low density and poor reactivity of active sites within its basal plane. Here, we report that domain boundaries in the basal plane of monolayer MoS2 can greatly enhance its hydrogen evolution reaction performance by serving as active sites. Two types of effective domain boundaries, the 2H-2H domain boundaries and the 2H-1T phase boundaries, were investigated. Superior hydrogen evolution reaction catalytic activity, long-term stability and universality in both acidic and alkaline conditions were achieved based on a multi-hierarchy design of these two types of domain boundaries. We further demonstrate that such superior catalysts are feasible at a large scale by applying this multi-hierarchy design of domain boundaries to wafer-scale monolayer MoS2 films.
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Conway B, Tilak B. Interfacial processes involving electrocatalytic evolution and oxidation of H2, and the role of chemisorbed H. Electrochim. Acta. 2002;47:3571–3594. doi: 10.1016/S0013-4686(02)00329-8. - DOI
Publication types
LinkOut - more resources
Full Text Sources

