Decoding the sweet regulation of apoptosis: the role of glycosylation and galectins in apoptotic signaling pathways
- PMID: 30903104
- PMCID: PMC6748114
- DOI: 10.1038/s41418-019-0317-6
Decoding the sweet regulation of apoptosis: the role of glycosylation and galectins in apoptotic signaling pathways
Abstract
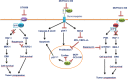
Glycosylation and glycan-binding proteins such as galectins play an important role in the control of cell death signaling. Strikingly, very little attention has been given so far to the understanding of the molecular details behind this key regulatory network. Glycans attached to the death receptors such as CD95 and TRAIL-Rs, either alone or in a complex with galectins, might promote or inhibit apoptotic signals. However, we have just started to decode the functions of galectins in the modulation of extrinsic and intrinsic apoptosis. In this work, we have discussed the current understanding of the glycosylation-galectin regulatory network in CD95- as well as TRAIL-R-induced apoptosis and therapeutic strategies based on targeting galectins in cancer.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

