A combinatorial biomarker predicts pathologic complete response to neoadjuvant lapatinib and trastuzumab without chemotherapy in patients with HER2+ breast cancer
- PMID: 30903140
- PMCID: PMC6594453
- DOI: 10.1093/annonc/mdz076
A combinatorial biomarker predicts pathologic complete response to neoadjuvant lapatinib and trastuzumab without chemotherapy in patients with HER2+ breast cancer
Abstract
Background: HER2-positive (+) breast cancers, defined by HER2 overexpression and/or amplification, are often addicted to HER2 to maintain their malignant phenotype. Yet, some HER2+ tumors do not benefit from anti-HER2 therapy. We hypothesize that HER2 amplification levels and PI3K pathway activation are key determinants of response to HER2-targeted treatments without chemotherapy.
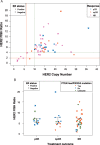
Patients and methods: Baseline HER2+ tumors from patients treated with neoadjuvant lapatinib plus trastuzumab [with endocrine therapy for estrogen receptor (ER)+ tumors] in TBCRC006 (NCT00548184) were evaluated in a central laboratory for HER2 amplification by fluorescence in situ hybridization (FISH) (n = 56). HER2 copy number (CN) and FISH ratios, and PI3K pathway status, defined by PIK3CA mutations or PTEN levels by immunohistochemistry were available for 41 tumors. Results were correlated with pathologic complete response (pCR; no residual invasive tumor in breast).
Results: Thirteen of the 56 patients (23%) achieved pCR. None of the 11 patients with HER2 ratio <4 and/or CN <10 achieved pCR, whereas 13/45 patients (29%) with HER2 ratio ≥4 and/or CN ≥10 attained pCR (P = 0.0513). Of the 18 patients with tumors expressing high PTEN or wild-type (WT) PIK3CA (intact PI3K pathway), 7 (39%) achieved pCR, compared with 1/23 (4%) with PI3K pathway alterations (P = 0.0133). Seven of the 16 patients (44%) with HER2 ratio ≥4 and intact PI3K pathway achieved pCR, whereas only 1/25 (4%) patients not meeting these criteria achieved pCR (P = 0.0031).
Conclusions: Our findings suggest that there is a clinical subtype in breast cancer with high HER2 amplification and intact PI3K pathway that is especially sensitive to HER2-targeted therapies without chemotherapy. A combination of HER2 FISH ratio and PI3K pathway status warrants validation to identify patients who may be treated with HER2-targeted therapy without chemotherapy.
Keywords: PIK3CA mutations; ErbB2 receptor tyrosine kinase; PTEN protein; breast cancer; fluorescent in situ hybridization; precision medicine.
© The Author(s) 2019. Published by Oxford University Press on behalf of the European Society for Medical Oncology. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures
References
-
- Arpino G, Gutierrez C, Weiss H. et al. Treatment of human epidermal growth factor receptor 2-overexpressing breast cancer xenografts with multiagent HER-targeted therapy. J Natl Cancer Inst 2007; 99(9): 694–705. - PubMed
-
- Rimawi MF, Mayer IA, Forero A. et al. Multicenter phase II study of neoadjuvant lapatinib and trastuzumab with hormonal therapy and without chemotherapy in patients with human epidermal growth factor receptor 2-overexpressing breast cancer: TBCRC 006. J Clin Oncol 2013; 31(14): 1726–1731. - PMC - PubMed
-
- Llombart-Cussac A, Cortes J, Pare L. et al. HER2-enriched subtype as a predictor of pathological complete response following trastuzumab and lapatinib without chemotherapy in early-stage HER2-positive breast cancer (PAMELA): an open-label, single-group, multicentre, phase 2 trial. Lancet Oncol 2017; 18(4): 545–554. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

