Delayed Treatment With 4-Methylpyrazole Protects Against Acetaminophen Hepatotoxicity in Mice by Inhibition of c-Jun n-Terminal Kinase
- PMID: 30903181
- PMCID: PMC6592188
- DOI: 10.1093/toxsci/kfz077
Delayed Treatment With 4-Methylpyrazole Protects Against Acetaminophen Hepatotoxicity in Mice by Inhibition of c-Jun n-Terminal Kinase
Abstract
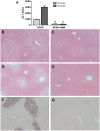
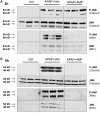
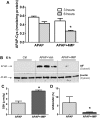
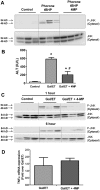
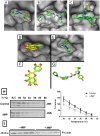
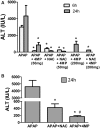
Acetaminophen (APAP) overdose is the most common cause of hepatotoxicity and acute liver failure in the United States and many western countries. However, the only clinically approved antidote, N-acetylcysteine, has a limited therapeutic window. 4-Methylpyrazole (4MP) is an antidote for methanol and ethylene glycol poisoning, and we have recently shown that cotreatment of 4MP with APAP effectively prevents toxicity by inhibiting Cyp2E1. To evaluate if 4MP can be used therapeutically, C57BL/6J mice were treated with 300 mg/kg APAP followed by 50 mg/kg 4MP 90 min later (after the metabolism phase). In these experiments, 4MP significantly attenuated liver injury at 3, 6, and 24 h after APAP as shown by 80%-90% reduction in plasma alanine aminotransferase activities and reduced areas of necrosis. 4MP prevented c-Jun c-Jun N-terminal kinase (JNK) activation and its mitochondrial translocation, and reduced mitochondrial oxidant stress and nuclear DNA fragmentation. 4MP also prevented JNK activation in other liver injury models. Molecular docking experiments showed that 4MP can bind to the ATP binding site of JNK. These data suggest that treatment with 4MP after the metabolism phase effectively prevents APAP-induced liver injury in the clinically relevant mouse model in vivo mainly through the inhibition of JNK activation. 4MP, a drug approved for human use, is as effective as N-acetylcysteine or can be even more effective in cases of severe overdoses with prolonged metabolism (600 mg/kg). 4MP acts on alternative therapeutic targets and thus may be a novel approach to treatment of APAP overdose in patients that complements N-acetylcysteine.
Keywords: N-acetylcysteine; acetaminophen hepatotoxicity; autophagy; c-Jun N-terminal kinase; galactosamine-endotoxin; mitochondria.
© The Author(s) 2019. Published by Oxford University Press on behalf of the Society of Toxicology. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures
References
-
- Adams P. D., Afonine P. V., Bunkoczi G., Chen V. B., Davis I. W., Echols N., Headd J. J., Hung L. W., Kapral G. J., Grosse-Kunstleve R. W., et al. (2010). PHENIX: A comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D. Biol. Crystallogr. 66, 213–221. - PMC - PubMed
-
- Bajt M. L., Cover C., Lemasters J. J., Jaeschke H. (2006). Nuclear translocation of endonuclease G and apoptosis-inducing factor during acetaminophen-induced liver cell injury. Toxicol. Sci. 94, 217–225. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

