NOD1 and NOD2 of the innate immune system is differently expressed in human clear cell renal cell carcinoma, corresponding healthy renal tissue, its vasculature and primary isolated renal tubular epithelial cells
- PMID: 30903318
- PMCID: PMC11810433
- DOI: 10.1007/s00432-019-02901-7
NOD1 and NOD2 of the innate immune system is differently expressed in human clear cell renal cell carcinoma, corresponding healthy renal tissue, its vasculature and primary isolated renal tubular epithelial cells
Abstract
Purpose: NOD1 and NOD2 (nucleotide-binding oligomerization domain)-receptors are intracellular receptors and belong to the family of pattern recognition receptors being present in both human and murine renal tubular cells. Besides, NOD1 has been proved to promote apoptosis, upon its overexpression. Hence, we aimed to investigate NOD1 and NOD2 expression in human clear cell renal cell carcinoma (ccRCC).
Methods: Tumor and corresponding adjacent healthy tissues from 41 patients with histopathological diagnosis of ccRCC as well as primary isolated renal tubular epithelial cells (TECs) and tumor tissue from a murine xenograft model using CAKI-1 ccRCC cells were analyzed.
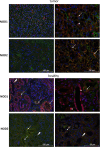
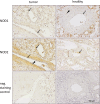
Results: NOD1 and NOD2 mRNA was constitutively expressed in both tumor and adjacent healthy renal tissue, with NOD1 being significantly lower and in contrast NOD2 significantly higher expressed in tumor tissue compared to healthy tissues. Immunohistochemically, NOD1 was located not only in the cytoplasm, but also in the nucleus in ccRCC tissue whereas NOD2 was solely localized in the cytoplasm in both human ccRCC as well as in the healthy tubular system. Focusing on the vasculature, NOD2 displayed broader expression than NOD1. In primary TECs as well as CAKI-1 cells NOD1 and NOD2 was constitutively expressed and increasable upon LPS stimulation. In the mouse xenograft model, human NOD1 mRNA was significantly higher expressed compared to NOD2. In contrast hereto, we observed a shift towards lower mouse NOD1 compared to NOD2 mRNA expression.
Conclusion: In view of reduced apoptosis-associated NOD1 expression in ccRCC tissue opposed to higher expression of NOD2 in tumor vasculature, inducibility of NOD expression in TECs as well as the detected shift of NOD1 and NOD2 expression in the mouse xenograft model, modulation of NOD receptors might, therefore, provide a molecular therapeutic approach in ccRCC.
Keywords: Clear cell renal cell carcinoma; Innate immunity; Kidney cancer; NLR.
Conflict of interest statement
The authors declare that they have no conflict of interest and that the results presented in this paper have not been published previously in whole or part.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

