Magnetic resonance imaging of noradrenergic neurons
- PMID: 30903359
- PMCID: PMC6509075
- DOI: 10.1007/s00429-019-01858-0
Magnetic resonance imaging of noradrenergic neurons
Abstract
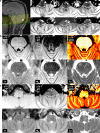
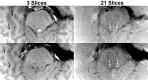
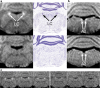
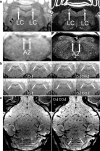
Noradrenaline is a neurotransmitter involved in general arousal, selective attention, memory, inflammation, and neurodegeneration. The purpose of this work was to delineate noradrenergic neurons in vivo by T1-weighted MRI with magnetization transfer (MT). In the brainstem of human and mice, MRI identified the locus coeruleus, dorsal motor vagus nucleus, and nucleus tractus solitarius. Given (1) the long T1 and low magnetization transfer ratio for the noradrenergic cell groups compared to other gray matter, (2) significant correlation between MT MRI signal intensity and proton density, and (3) no correlation between magnetization transfer ratio (or R1) and iron, copper, or manganese in human brain, the high MRI signal of the noradrenergic neurons must be attributed to abundant water protons interacting with any T1-shortening paramagnetic ions in active cells rather than to specific T1-shortening molecules. The absence of a high MRI signal from the locus coeruleus of Ear2(-/-) mice lacking noradrenergic neurons confirms that cell bodies of noradrenergic neurons are the source of the bright MRI appearance. The observation of this high signal in DBH(-/-) mice, in 3-week-old mice, and in mice under hyperoxia/hypercapnia/hypoxia together with the general absence of neuromelanin (NM) in noradrenergic neurons of young rodents further excludes that it is due to NM, dopamine β-hydroxylase, their binding to paramagnetic ions, blood inflow, or hemoglobin. Instead, these findings indicate a high density of water protons whose T1 is shortened by paramagnetic ions as the relevant source of the high MRI signal. In the brain of APP/PS1/Ear2(-/-) mice, a transgenic model of Alzheimer's disease, MRI detected noradrenergic neuron loss in the locus coeruleus. Proton magnetic resonance spectroscopy revealed that a 60-75% reduction of noradrenaline is responsible for a reduction of N-acetylaspartate and glutamate in the hippocampus as well as for a shortening of the water proton T2 in the frontal cortex. These results suggest that a concurrent shortage of noradrenaline in Alzheimer's disease accelerates pathologic processes such as inflammation and neuron loss.
Keywords: Alzheimer’s disease; Dorsal motor vagus nucleus; Locus coeruleus; Magnetization transfer; Neuromelanin; Nucleus tractus solitarius.
Conflict of interest statement
Conflict of interest
The authors declare that they have no competing financial interests.
Ethical standards
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
All participants gave written informed consent before each examination.
Human/animal rights statement
All animal experiments were performed in accordance with German animal protection laws after approval by the responsible governmental authority.
Figures



Similar articles
-
Magnetic resonance imaging of brain cell water.Sci Rep. 2019 Mar 25;9(1):5084. doi: 10.1038/s41598-019-41587-2. Sci Rep. 2019. PMID: 30911100 Free PMC article. Review.
-
High-resolution in vivo imaging of human locus coeruleus by magnetization transfer MRI at 3T and 7T.Neuroimage. 2018 Mar;168:427-436. doi: 10.1016/j.neuroimage.2017.07.045. Epub 2017 Jul 22. Neuroimage. 2018. PMID: 28743460
-
Examining the Role of the Noradrenergic Locus Coeruleus for Predicting Attention and Brain Maintenance in Healthy Old Age and Disease: An MRI Structural Study for the Alzheimer's Disease Neuroimaging Initiative.Cells. 2021 Jul 20;10(7):1829. doi: 10.3390/cells10071829. Cells. 2021. PMID: 34359997 Free PMC article.
-
Microstructural integrity of the locus coeruleus and its tracts reflect noradrenergic degeneration in Alzheimer's disease and Parkinson's disease.Transl Neurodegener. 2024 Feb 9;13(1):9. doi: 10.1186/s40035-024-00400-5. Transl Neurodegener. 2024. PMID: 38336865 Free PMC article.
-
Exploring the Role of Locus Coeruleus in Alzheimer's Disease: a Comprehensive Update on MRI Studies and Implications.Curr Neurol Neurosci Rep. 2023 Dec;23(12):925-936. doi: 10.1007/s11910-023-01324-9. Epub 2023 Dec 8. Curr Neurol Neurosci Rep. 2023. PMID: 38064152 Free PMC article. Review.
Cited by
-
Locus coeruleus MRI contrast, cerebral perfusion, and plasma Alzheimer's disease biomarkers in older adults.Neurobiol Aging. 2025 Mar;147:12-21. doi: 10.1016/j.neurobiolaging.2024.11.008. Epub 2024 Nov 27. Neurobiol Aging. 2025. PMID: 39637519
-
Locus Coeruleus Magnetic Resonance Imaging in Neurological Diseases.Curr Neurol Neurosci Rep. 2020 Dec 12;21(1):2. doi: 10.1007/s11910-020-01087-7. Curr Neurol Neurosci Rep. 2020. PMID: 33313963 Free PMC article. Review.
-
Application of Neuromelanin MR Imaging in Parkinson Disease.J Magn Reson Imaging. 2023 Feb;57(2):337-352. doi: 10.1002/jmri.28414. Epub 2022 Aug 26. J Magn Reson Imaging. 2023. PMID: 36017746 Free PMC article. Review.
-
State-of-the-art imaging of neuromodulatory subcortical systems in aging and Alzheimer's disease: Challenges and opportunities.Neurosci Biobehav Rev. 2023 Jan;144:104998. doi: 10.1016/j.neubiorev.2022.104998. Epub 2022 Dec 13. Neurosci Biobehav Rev. 2023. PMID: 36526031 Free PMC article. Review.
-
Noradrenergic modulation of rhythmic neural activity shapes selective attention.Trends Cogn Sci. 2022 Jan;26(1):38-52. doi: 10.1016/j.tics.2021.10.009. Epub 2021 Nov 16. Trends Cogn Sci. 2022. PMID: 34799252 Free PMC article. Review.
References
-
- Burton DR, Forsen S, Karlstrom G, Dwek RA. Proton relaxation enhancement (PRE) in biochemistry: a critical survey. Prog Nucl Magn Reson Spectrosc. 1979;13:1–45. doi: 10.1016/0079-6565(79)80012-6. - DOI
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

