The Antibodiome-Mapping the Humoral Immune Response to HIV
- PMID: 30903381
- PMCID: PMC6441398
- DOI: 10.1007/s11904-019-00432-x
The Antibodiome-Mapping the Humoral Immune Response to HIV
Abstract
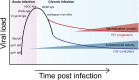
Purpose of review: The design of an HIV vaccine remains an elusive but top priority. Data from the non-human primate model and the first moderately protective HIV vaccine trial (RV144) point to a role for qualitative changes in humoral immune functions in protection from infection. Here, we review the current understanding of the antibody response throughout HIV infection, the known correlates of protection, and current strategies to manipulate antibodies to put an end to the epidemic.
Recent findings: Recent studies point to innate immune-recruiting antibody function in preventing infection as well as controlling viremia following infection. These data have begun to inform next-generation design of HIV vaccines and antibody therapies by uncovering new viral targets and antibody architectures to improve potency and breadth. Emerging data illustrate a role for innate immune recruiting-antibodies in conferring protection against HIV infection as well as promoting viral control and clearance, offering an unprecedented opportunity to modulate and improve antibody function to fight HIV more effectively.
Keywords: Antibodies; HIV-1; Innate immunity; Natural HIV control; Vaccination.
Conflict of interest statement
Conflict of Interest
The authors declare that they have no conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Figures

References
-
- Sok D, Pauthner M, Briney B, Lee JH, Saye-Francisco KL, Hsueh J, Ramos A, le KM, Jones M, Jardine JG, Bastidas R, Sarkar A, Liang CH, Shivatare SS, Wu CY, Schief WR, Wong CH, Wilson IA, Ward AB, Zhu J, Poignard P, Burton DR. A prominent site of antibody vulnerability on HIV envelope incorporates a motif associated with CCR5 binding and its camouflaging glycans. Immunity. 2016;45(1):31–45. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

