Allergic Comorbidity in Eosinophilic Esophagitis: Mechanistic Relevance and Clinical Implications
- PMID: 30903437
- PMCID: PMC6626558
- DOI: 10.1007/s12016-019-08733-0
Allergic Comorbidity in Eosinophilic Esophagitis: Mechanistic Relevance and Clinical Implications
Abstract
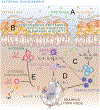
Allergic eosinophilic esophagitis (EoE) is a chronic, allergen-mediated inflammatory disease of the esophagus, and the most common cause of prolonged dysphagia in children and young adults in the developed world. While initially undistinguished from gastroesophageal reflux disease-associated esophageal eosinophilia, EoE is now recognized as a clinically distinct entity that shares fundamental inflammatory features of other allergic conditions and is similarly increasing in incidence and prevalence. The clinical and epidemiologic associations between EoE and other allergic manifestations are well established. In addition to exaggerated rates of atopic dermatitis, IgE-mediated food allergy, asthma, and allergic rhinitis in EoE patients, each of these allergic manifestations imparts individual and cumulative risk for subsequent EoE diagnosis. As such, EoE may be a member of the "allergic march"-the natural history of allergic manifestations during childhood. Several determinants likely contribute to the relationship between these conditions, including shared genetic, environmental, and immunologic factors. Herein, we present a comprehensive review of allergic comorbidity in EoE. We discuss areas of the genome associated with both EoE and other allergic diseases, including the well-studied variants encoding thymic stromal lymphopoietin and calpain 14, among other "atopic" regions. We summarize ways that environmental factors (such as microbiome-altering pressures and aeroallergen exposure) may predispose to multiple allergic conditions including EoE. Finally, we touch on some fundamental features of type 2 inflammation, and the resulting implications for the development of multiple allergic manifestations. We conclude with an analysis of the "type 2" biologics, and how mechanistic similarities between EoE and the other allergic manifestations have important implications for screening and treatment of the allergic patient.
Keywords: Allergic rhinitis; Asthma; Atopic dermatitis; Eosinophilic esophagitis; Food allergy.
Conflict of interest statement
Figures


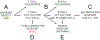
References
-
- Cianferoni A, Spergel J. Eosinophilic esophagitis: A comprehensive review. Clin Rev Allergy Immunol. 2016;50(2): 159–74. - PubMed
-
- Breier-Mackie S Cultural competence and patient advocacy: The new challenge for nurses. Gastroenterol Nurs. 2007;30(2): 120–2. - PubMed
-
- Picus D FP. Eosinophilic esophagitis. AJR Am J Roentgenol. 1981;136(5): 1001–3. - PubMed
-
- Walsh S V, Antonioli DA, Goldman H, Fox VL, Bousvaros A, Leichtner AM, et al. Allergic esophagitis in children: A clinicopathological entity. Am J Surg Pathol. 1999;23(4):390–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

