Wnt and RUNX2 mediate cartilage breakdown by osteoarthritis synovial fibroblast-derived ADAMTS-7 and -12
- PMID: 30903650
- PMCID: PMC6533528
- DOI: 10.1111/jcmm.14283
Wnt and RUNX2 mediate cartilage breakdown by osteoarthritis synovial fibroblast-derived ADAMTS-7 and -12
Abstract
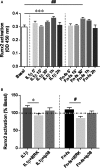
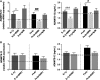
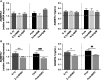
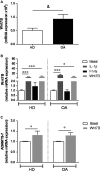
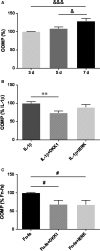
Failure of therapeutic approaches for the treatment of osteoarthritis (OA) based on the inhibition of metalloproteinases, might be because of their constitutive expression in homeostasis, together with their network complexity. The knowledge of this network would contribute to selective target pathological conditions. In this sense, blockade of mediators produced by neighbouring joint cells, such as synovial fibroblasts (SF), would prevent cartilage damage. Thus, we studied the contribution of ADAMTS-7 and -12 from SF to cartilage oligomeric matrix protein (COMP) degradation, and the signalling pathways involved in their expression. We report for the first time in SF, the involvement of ERK-Runx2 axis and Wnt/β-catenin signalling in ADAMTS-12 and ADAMTS-7 expressions, respectively, with the subsequent consequences in COMP degradation from cartilage extracellular matrix. After stimulation with IL-1β or fibronectin fragments, we showed that ERK inhibition decreased Runx2 activation and ADAMTS-12 expression in OA-SF, also reducing Fn-fs-induced COMP degradation. Blockage of Wnt signalling by DKK1 reduced ADAMTS-7 and COMP degradation in OA-SF as well. In addition, Wnt7B expression was induced by IL-1β and by itself, also increasing ADAMTS-7. Our results could contribute to the development of disease-modifying OA drugs targeting ADAMTS-7 and -12 for the prevention of extracellular matrix components degradation like COMP.
Keywords: ADAMTS-12; ADAMTS-7; COMP; Runx2; Wnt signalling; extracellular matrix; osteoarthritis; synovial fibroblasts.
© 2019 The Authors. Journal of Cellular and Molecular Medicine published by John Wiley & Sons Ltd and Foundation for Cellular and Molecular Medicine.
Conflict of interest statement
The authors confirm that there are no conflict of interest.
Figures

Similar articles
-
VIP and CRF reduce ADAMTS expression and function in osteoarthritis synovial fibroblasts.J Cell Mol Med. 2016 Apr;20(4):678-87. doi: 10.1111/jcmm.12777. Epub 2016 Jan 28. J Cell Mol Med. 2016. PMID: 26818776 Free PMC article.
-
Healthy and Osteoarthritic Synovial Fibroblasts Produce a Disintegrin and Metalloproteinase with Thrombospondin Motifs 4, 5, 7, and 12: Induction by IL-1β and Fibronectin and Contribution to Cartilage Damage.Am J Pathol. 2016 Sep;186(9):2449-61. doi: 10.1016/j.ajpath.2016.05.017. Epub 2016 Jul 20. Am J Pathol. 2016. PMID: 27449198
-
miR-105/Runx2 axis mediates FGF2-induced ADAMTS expression in osteoarthritis cartilage.J Mol Med (Berl). 2016 Jun;94(6):681-94. doi: 10.1007/s00109-016-1380-9. Epub 2016 Jan 27. J Mol Med (Berl). 2016. PMID: 26816250
-
Cartilage Oligomeric Matrix Protein: Matricellular and Matricrine Signaling in Cardiovascular Homeostasis and Disease.Curr Vasc Pharmacol. 2017;15(3):186-196. doi: 10.2174/1570161115666170201121232. Curr Vasc Pharmacol. 2017. PMID: 28155614 Review.
-
The Mechanism and Role of ADAMTS Protein Family in Osteoarthritis.Biomolecules. 2022 Jul 8;12(7):959. doi: 10.3390/biom12070959. Biomolecules. 2022. PMID: 35883515 Free PMC article. Review.
Cited by
-
Exosomes: roles and therapeutic potential in osteoarthritis.Bone Res. 2020 Jun 19;8:25. doi: 10.1038/s41413-020-0100-9. eCollection 2020. Bone Res. 2020. PMID: 32596023 Free PMC article.
-
BAY-9835: Discovery of the First Orally Bioavailable ADAMTS7 Inhibitor.J Med Chem. 2024 Feb 22;67(4):2907-2940. doi: 10.1021/acs.jmedchem.3c02036. Epub 2024 Feb 13. J Med Chem. 2024. PMID: 38348661 Free PMC article.
-
Wnt signaling: a promising target for osteoarthritis therapy.Cell Commun Signal. 2019 Aug 16;17(1):97. doi: 10.1186/s12964-019-0411-x. Cell Commun Signal. 2019. PMID: 31420042 Free PMC article. Review.
-
The potential role of synovial cells in the progression and treatment of osteoarthritis.Exploration (Beijing). 2023 Jul 10;3(5):20220132. doi: 10.1002/EXP.20220132. eCollection 2023 Oct. Exploration (Beijing). 2023. PMID: 37933282 Free PMC article. Review.
-
Xanthohumol Inhibited Mechanical Stimulation-Induced Articular ECM Degradation by Mediating lncRNA GAS5/miR-27a Axis.Front Pharmacol. 2021 Sep 10;12:737552. doi: 10.3389/fphar.2021.737552. eCollection 2021. Front Pharmacol. 2021. PMID: 34616299 Free PMC article.
References
-
- Bijlsma JW, Berenbaum F, Lafeber FP. Osteoarthritis: an update with relevance for clinical practice. Lancet. 2011;377:2115‐2126. - PubMed
-
- Rodriguez‐Lopez J, Mustafa Z, Pombo‐Suarez M, et al. Genetic variation including nonsynonymous polymorphisms of a major aggrecanase, ADAMTS‐5, in susceptibility to osteoarthritis. Arthritis Rheum. 2008;58:435‐441. - PubMed
-
- Pérez‐García S, Gutiérrez‐Cañas I, Seoane IV, et al. Healthy and osteoarthritic synovial fibroblasts produce a disintegrin and metalloproteinase with thrombospondin motifs 4, 5, 7, and 12: induction by IL‐1beta and fibronectin and contribution to cartilage damage. Am J Pathol. 2016;186:2449‐2461. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

