Electrophysiological differences between upper and lower limb movements in the human subthalamic nucleus
- PMID: 30903826
- PMCID: PMC6487671
- DOI: 10.1016/j.clinph.2019.02.011
Electrophysiological differences between upper and lower limb movements in the human subthalamic nucleus
Abstract
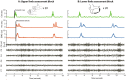
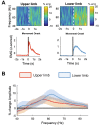
Objective: Functional processes in the brain are segregated in both the spatial and spectral domain. Motivated by findings reported at the cortical level in healthy participants we test the hypothesis in the basal ganglia of Parkinson's disease patients that lower frequency beta band activity relates to motor circuits associated with the upper limb and higher beta frequencies with lower limb movements.
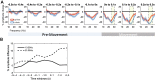
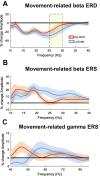
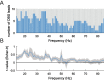
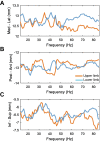
Methods: We recorded local field potentials (LFPs) from the subthalamic nucleus using segmented "directional" DBS leads, during which patients performed repetitive upper and lower limb movements. Movement-related spectral changes in the beta and gamma frequency-ranges and their spatial distributions were compared between limbs.
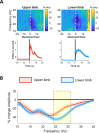
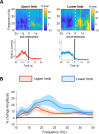
Results: We found that the beta desynchronization during leg movements is characterised by a strikingly greater involvement of higher beta frequencies (24-31 Hz), regardless of whether this was contralateral or ipsilateral to the limb moved. The spatial distribution of limb-specific movement-related changes was evident at higher gamma frequencies.
Conclusion: Limb processing in the basal ganglia is differentially organised in the spectral and spatial domain and can be captured by directional DBS leads.
Significance: These findings may help to refine the use of the subthalamic LFPs as a control signal for adaptive DBS and neuroprosthetic devices.
Keywords: Basal ganglia; Directional deep brain stimulation; Local field potentials; Motor network; Somatotopy.
Copyright © 2019 International Federation of Clinical Neurophysiology. Published by Elsevier B.V. All rights reserved.
Figures
Similar articles
-
Subthalamic nucleus activity dynamics and limb movement prediction in Parkinson's disease.Brain. 2020 Feb 1;143(2):582-596. doi: 10.1093/brain/awz417. Brain. 2020. PMID: 32040563 Free PMC article.
-
Beta-band oscillations in the supplementary motor cortex are modulated by levodopa and associated with functional activity in the basal ganglia.Neuroimage Clin. 2018 May 18;19:559-571. doi: 10.1016/j.nicl.2018.05.021. eCollection 2018. Neuroimage Clin. 2018. PMID: 29984164 Free PMC article.
-
Impact of Subthalamic Deep Brain Stimulation Frequency on Upper Limb Motor Function in Parkinson's Disease.J Parkinsons Dis. 2018;8(2):267-271. doi: 10.3233/JPD-171150. J Parkinsons Dis. 2018. PMID: 29614696 Free PMC article. Clinical Trial.
-
Eye movements and deep brain stimulation.Curr Opin Neurol. 2016 Feb;29(1):69-73. doi: 10.1097/WCO.0000000000000276. Curr Opin Neurol. 2016. PMID: 26641812 Review.
-
[Emotion and basal ganglia (II): what can we learn from subthalamic nucleus deep brain stimulation in Parkinson's disease?].Rev Neurol (Paris). 2012 Aug-Sep;168(8-9):642-8. doi: 10.1016/j.neurol.2012.06.012. Epub 2012 Aug 17. Rev Neurol (Paris). 2012. PMID: 22901366 Review. French.
Cited by
-
Mechanisms of Network Interactions for Flexible Cortico-Basal Ganglia-Mediated Action Control.eNeuro. 2021 Jun 11;8(3):ENEURO.0009-21.2021. doi: 10.1523/ENEURO.0009-21.2021. Print 2021 May-Jun. eNeuro. 2021. PMID: 33883192 Free PMC article.
-
Long-term consistency of aperiodic and periodic physiomarkers in subthalamic local field potentials in Parkinson's disease.NPJ Parkinsons Dis. 2025 Jul 10;11(1):204. doi: 10.1038/s41531-025-01053-5. NPJ Parkinsons Dis. 2025. PMID: 40640153 Free PMC article.
-
Clinical neurophysiological interrogation of motor slowing: A critical step towards tuning adaptive deep brain stimulation.Clin Neurophysiol. 2023 Aug;152:43-56. doi: 10.1016/j.clinph.2023.04.013. Epub 2023 May 16. Clin Neurophysiol. 2023. PMID: 37285747 Free PMC article.
-
Subthalamic high-beta oscillation informs the outcome of deep brain stimulation in patients with Parkinson's disease.Front Hum Neurosci. 2022 Sep 8;16:958521. doi: 10.3389/fnhum.2022.958521. eCollection 2022. Front Hum Neurosci. 2022. PMID: 36158623 Free PMC article.
-
Combining Multimodal Biomarkers to Guide Deep Brain Stimulation Programming in Parkinson Disease.Neuromodulation. 2023 Feb;26(2):320-332. doi: 10.1016/j.neurom.2022.01.017. Epub 2022 Feb 24. Neuromodulation. 2023. PMID: 35219571 Free PMC article.
References
-
- Androulidakis A.G., Doyle L.M.F., Gilbertson T.P., Brown P. Corrective movements in response to displacements in visual feedback are more effective during periods of 13–35 Hz oscillatory synchrony in the human corticospinal system. Eur J Neurosci. 2006;24:3299–3304. - PubMed
-
- Babiloni C., Babiloni F., Carducci F., Cincotti F., Cocozza G., Del Percio C. Human cortical electroencephalography (EEG) rhythms during the observation of simple aimless movements: a high-resolution EEG study. Neuroimage. 2002;17:559–572. - PubMed
-
- Brown P. Oscillatory nature of human basal ganglia activity: relationship to the pathophysiology of Parkinson’s disease. Mov Disord. 2003;18:357–363. - PubMed

