INSoles To Ease Pressure (INSTEP) Study: a multicentre, randomised controlled feasibility study to compare the effectiveness of a novel instant optimised insole with a standard insole for people with diabetic neuropathy: a study protocol
- PMID: 30904880
- PMCID: PMC6477388
- DOI: 10.1136/bmjopen-2019-029185
INSoles To Ease Pressure (INSTEP) Study: a multicentre, randomised controlled feasibility study to compare the effectiveness of a novel instant optimised insole with a standard insole for people with diabetic neuropathy: a study protocol
Abstract
Introduction: Foot ulceration is a multifactorial complication of diabetes. Therapeutic insoles and footwear are frequently used to reduce elevated tissue pressures associated with risk of foot ulceration. A novel protocol using in-shoe pressure measurement technology to provide an instant optimised insole and house shoe solution has been developed, with the aim of reducing foot ulceration.
Aim: This study aims to assess the feasibility of conducting a multicentre randomised controlled trial to compare the effectiveness of a novel instant optimised insole with a standard insole for people with diabetic neuropathy.
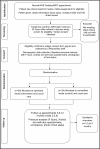
Methods and analysis: This study is a participant and assessor blinded, randomised, multicentre parallel group feasibility trial with embedded qualitative study. Seventy-six participants will be recruited from three podiatry clinics and randomised to an optimised insole plus usual care (intervention group) or standard insole plus usual care (control group) using a minimisation by randomisation procedure by study centre and previous ulcer status. Assessment visits and data collection will be at baseline, 3 months, 6 months and 12 months. Feasibility and acceptability of the trial procedures will be determined in terms of recruitment and retention rates, data completion rates, intervention adherence and effectiveness of the blinding.Assessment of the appropriateness and performance of outcome measures will inform selection of the primary and secondary outcomes and sample size estimate for the anticipated definitive randomised controlled trial. Clinical outcomes include incidence of plantar foot ulceration and change in peak plantar pressure. Twelve participants (four from each centre) and three treating podiatrists (one from each centre) will be interviewed to explore their experiences of receiving and delivering the intervention.
Ethics and dissemination: The study was approved by the South-West Exeter Research Ethics Committee. Findings will be disseminated through conference presentations, public platforms and academic publications.
Trials registration number: ISRCTN16011830; Pre-results.
Keywords: diabetic foot; primary care; qualitative research.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
References
-
- Driver VR, Fabbi M, Lavery LA, et al. The costs of diabetic foot: the economic case for the limb salvage team. J Am Podiatr Med Assoc 2010;100:335–41. - PubMed
-
- Van Dieren S, Beulens JW, van der S, et al. The global burden of diabetes and its complications: an emerging pandemic. Eur J Cardiovasc Prev Rehabil rehabilitation 2010;17:s3–8. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical