2-Aminoindan and its ring-substituted derivatives interact with plasma membrane monoamine transporters and α2-adrenergic receptors
- PMID: 30904940
- PMCID: PMC6848746
- DOI: 10.1007/s00213-019-05207-1
2-Aminoindan and its ring-substituted derivatives interact with plasma membrane monoamine transporters and α2-adrenergic receptors
Abstract
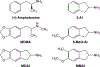
Rationale: Over the last decade, many new psychostimulant analogues have appeared on the recreational drug market and most are derivatives of amphetamine or cathinone. Another class of designer drugs is derived from the 2-aminoindan structural template. Several members of this class, including the parent compound 2-aminoindan (2-AI), have been sold as designer drugs. Another aminoindan derivative, 5-methoxy-2-aminoindan (5-MeO-AI or MEAI), is the active ingredient in a product marketed online as an alcohol substitute.
Methods: Here, we tested 2-AI and its ring-substituted derivatives 5-MeO-AI, 5-methoxy-6-methyl-2-aminoindan (MMAI), and 5,6-methylenedioxy-2-aminoindan (MDAI) for their abilities to interact with plasma membrane monoamine transporters for dopamine (DAT), norepinephrine (NET) and serotonin (SERT). We also compared the binding affinities of the aminoindans at 29 receptor and transporter binding sites.
Results: 2-AI was a selective substrate for NET and DAT. Ring substitution increased potency at SERT while reducing potency at DAT and NET. MDAI was moderately selective for SERT and NET, with tenfold weaker effects on DAT. 5-MeO-AI exhibited some selectivity for SERT, having sixfold lower potency at NET and 20-fold lower potency at DAT. MMAI was highly selective for SERT, with 100-fold lower potency at NET and DAT. The aminoindans had relatively high affinity for α2-adrenoceptor subtypes. 2-AI had particularly high affinity for α2C receptors (Ki = 41 nM) and slightly lower affinity for the α2A (Ki = 134 nM) and α2B (Ki = 211 nM) subtypes. 5-MeO-AI and MMAI also had moderate affinity for the 5-HT2B receptor.
Conclusions: 2-AI is predicted to have (+)-amphetamine-like effects and abuse potential whereas the ring-substituted derivatives may produce 3,4-methylenedioxymethamphetamine (MDMA)-like effects but with less abuse liability.
Keywords: Analgesia; Binding; Dopamine; MEAI; Norepinephrine; Serotonin; Stimulant; Synaptosomes.
Conflict of interest statement
Figures

Similar articles
-
Comparison of the monoamine transporters from human and mouse in their sensitivities to psychostimulant drugs.BMC Pharmacol. 2006 Mar 3;6:6. doi: 10.1186/1471-2210-6-6. BMC Pharmacol. 2006. PMID: 16515684 Free PMC article.
-
The profile of mephedrone on human monoamine transporters differs from 3,4-methylenedioxymethamphetamine primarily by lower potency at the vesicular monoamine transporter.Eur J Pharmacol. 2015 May 15;755:119-26. doi: 10.1016/j.ejphar.2015.03.004. Epub 2015 Mar 11. Eur J Pharmacol. 2015. PMID: 25771452
-
MDMA (Ecstasy) and human dopamine, norepinephrine, and serotonin transporters: implications for MDMA-induced neurotoxicity and treatment.Psychopharmacology (Berl). 2007 Jan;189(4):489-503. doi: 10.1007/s00213-005-0174-5. Epub 2005 Oct 12. Psychopharmacology (Berl). 2007. PMID: 16220332
-
Pharmacology of MDMA- and Amphetamine-Like New Psychoactive Substances.Handb Exp Pharmacol. 2018;252:143-164. doi: 10.1007/164_2018_113. Handb Exp Pharmacol. 2018. PMID: 29633178 Review.
-
Using Ca2+-channel biosensors to profile amphetamines and cathinones at monoamine transporters: electro-engineering cells to detect potential new psychoactive substances.Psychopharmacology (Berl). 2019 Mar;236(3):973-988. doi: 10.1007/s00213-018-5103-5. Epub 2018 Nov 17. Psychopharmacology (Berl). 2019. PMID: 30448989 Free PMC article. Review.
Cited by
-
5-Methoxy-2-aminoindane Reverses Diet-Induced Obesity and Improves Metabolic Parameters in Mice: A Potential New Class of Antiobesity Therapeutics.ACS Pharmacol Transl Sci. 2024 Jul 30;7(8):2527-2543. doi: 10.1021/acsptsci.4c00353. eCollection 2024 Aug 9. ACS Pharmacol Transl Sci. 2024. PMID: 39144560 Free PMC article.
-
Beyond ecstasy: Alternative entactogens to 3,4-methylenedioxymethamphetamine with potential applications in psychotherapy.J Psychopharmacol. 2021 May;35(5):512-536. doi: 10.1177/0269881120920420. Epub 2020 Sep 10. J Psychopharmacol. 2021. PMID: 32909493 Free PMC article. Review.
References
-
- Anonymous (2017) 2-Aminoindan reports. Available online: https://erowid.org/experiences/subs/exp_2Aminoindan.shtml [Accessed: April 3, 2018]
-
- Auclair AL, Cathala A, Sarrazin F, Depoortere R, Piazza PV, Newman-Tancredi A, Spampinato U (2010) The central serotonin 2B receptor: a new pharmacological target to modulate the mesoaccumbens dopaminergic pathway activity. J Neurochem 114: 1323–32. - PubMed
-
- Battaglia G, Brooks BP, Kulsakdinun C, De Souza EB (1988) Pharmacologic profile of MDMA (3,4-methylenedioxymethamphetamine) at various brain recognition sites. Eur J Pharmacol 149: 159–63. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

