Eculizumab improves fatigue in refractory generalized myasthenia gravis
- PMID: 30905021
- PMCID: PMC6620379
- DOI: 10.1007/s11136-019-02148-2
Eculizumab improves fatigue in refractory generalized myasthenia gravis
Erratum in
-
Correction to: Eculizumab improves fatigue in refractory generalized myasthenia gravis.Qual Life Res. 2019 Aug;28(8):2255. doi: 10.1007/s11136-019-02204-x. Qual Life Res. 2019. PMID: 31115842 Free PMC article.
Abstract
Purpose: To evaluate the effect of eculizumab on perceived fatigue in patients with anti-acetylcholine receptor antibody-positive, refractory, generalized myasthenia gravis (MG) using the Quality of Life in Neurological Disorders (Neuro-QOL) Fatigue subscale, and to evaluate correlations between improvements in Neuro-QOL Fatigue and other clinical endpoints.
Methods: Neuro-QOL Fatigue, MG Activities of Daily Living (MG-ADL), Quantitative MG (QMG), and the 15-item MG Quality of Life (MG-QOL15) scales were administered during the phase 3, randomized, placebo-controlled REGAIN study (eculizumab, n = 62; placebo, n = 63) and subsequent open-label extension (OLE). Data were analyzed using repeated-measures models. Correlations between changes in Neuro-QOL Fatigue and in MG-ADL, QMG, and MG-QOL15 scores were determined at REGAIN week 26.
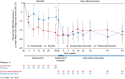
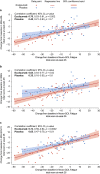
Results: At REGAIN week 26, eculizumab-treated patients showed significantly greater improvements in Neuro-QOL Fatigue scores than placebo-treated patients (consistent with improvements in MG-ADL, QMG, and MG-QOL15 scores previously reported in REGAIN). Improvements with eculizumab were sustained through OLE week 52. Correlations between Neuro-QOL Fatigue and MG-QOL15, MG-ADL, and QMG scores were strong for eculizumab-treated patients at REGAIN week 26, and strong, moderate, and weak, respectively, for placebo-treated patients.
Conclusions: Compared with placebo, eculizumab was associated with improvements in perceived fatigue that strongly correlated with improvements in MG-specific outcome measures. Trial ID Registration: NCT01997229, NCT02301624.
Keywords: Complement; Eculizumab; Fatigue; Myasthenia gravis; Neuro-QOL Fatigue; Quality of life; Terminal complement inhibition.
Conflict of interest statement
Henning Andersen, MD, DMSci, PhD, has received research and travel support, and speaker honoraria from CSL Behring, Eisai, Octapharma, Pfizer, Sanofi Genzyme and UCB Pharma and has served as a consultant on advisory boards for NMD Pharma, UCB Pharma and Sanofi Genzyme. Renato Mantegazza, MD, received funding for research and congress participation from Bayer, BioMarin, Sanofi Genzyme and Teva, and participated in Scientific Advisory Boards for Alexion Pharmaceuticals, Argenx BVBA and BioMarin. Jing Jing Wang, MD, was formerly employed by, and owns stock in, Alexion Pharmaceuticals. Fanny O’Brien, PhD, and Kaushik Patra, PhD, are employees of, and own stock in, Alexion Pharmaceuticals. James F. Howard, Jr, MD, obtained: research support from Alexion Pharmaceuticals during the conduct of the study; grants from Alexion Pharmaceuticals; research support from Research Triangle Institute/Centers for Disease Control and Prevention; grants from National Institutes of Health (including the National Institute of Neurological Disorders and Stroke, National Institute of Arthritis and Musculoskeletal and Skin Diseases); research support from the Muscular Dystrophy Association; honoraria from Alexion Pharmaceuticals, Argenx BVBA and Ra Pharmaceuticals; and non-financial support from Alexion Pharmaceuticals, Argenx BVBA, Ra Pharmaceuticals and Toleranzia AB, outside of the submitted work.
Figures
Similar articles
-
'Minimal symptom expression' in patients with acetylcholine receptor antibody-positive refractory generalized myasthenia gravis treated with eculizumab.J Neurol. 2020 Jul;267(7):1991-2001. doi: 10.1007/s00415-020-09770-y. Epub 2020 Mar 18. J Neurol. 2020. PMID: 32189108 Free PMC article. Clinical Trial.
-
Long-term efficacy and safety of eculizumab in Japanese patients with generalized myasthenia gravis: A subgroup analysis of the REGAIN open-label extension study.J Neurol Sci. 2019 Dec 15;407:116419. doi: 10.1016/j.jns.2019.08.004. Epub 2019 Aug 3. J Neurol Sci. 2019. PMID: 31698177
-
Safety and efficacy of eculizumab in anti-acetylcholine receptor antibody-positive refractory generalised myasthenia gravis (REGAIN): a phase 3, randomised, double-blind, placebo-controlled, multicentre study.Lancet Neurol. 2017 Dec;16(12):976-986. doi: 10.1016/S1474-4422(17)30369-1. Epub 2017 Oct 20. Lancet Neurol. 2017. PMID: 29066163 Clinical Trial.
-
Eculizumab: A Review in Generalized Myasthenia Gravis.Drugs. 2018 Mar;78(3):367-376. doi: 10.1007/s40265-018-0875-9. Drugs. 2018. PMID: 29435915 Free PMC article. Review.
-
Eculizumab treatment for myasthenia gravis subgroups: 2021 update.J Neuroimmunol. 2022 Jan 15;362:577767. doi: 10.1016/j.jneuroim.2021.577767. Epub 2021 Nov 18. J Neuroimmunol. 2022. PMID: 34823117 Review.
Cited by
-
Myasthenia gravis: the changing treatment landscape in the era of molecular therapies.Nat Rev Neurol. 2024 Feb;20(2):84-98. doi: 10.1038/s41582-023-00916-w. Epub 2024 Jan 8. Nat Rev Neurol. 2024. PMID: 38191918 Review.
-
Antibody Therapies in Autoimmune Neuromuscular Junction Disorders: Approach to Myasthenic Crisis and Chronic Management.Neurotherapeutics. 2022 Apr;19(3):897-910. doi: 10.1007/s13311-022-01181-3. Epub 2022 Feb 14. Neurotherapeutics. 2022. PMID: 35165857 Free PMC article. Review.
-
Determinants of quality of life in elderly rehabilitation users at a day care service center.J Phys Ther Sci. 2023 Jan;35(1):12-17. doi: 10.1589/jpts.35.12. Epub 2023 Jan 1. J Phys Ther Sci. 2023. PMID: 36628134 Free PMC article.
-
Outcome Measures in Clinical Trials of Patients With Myasthenia Gravis.Front Neurol. 2020 Dec 23;11:596382. doi: 10.3389/fneur.2020.596382. eCollection 2020. Front Neurol. 2020. PMID: 33424747 Free PMC article. Review.
-
Improvement of fatigue in generalised myasthenia gravis with zilucoplan.J Neurol. 2024 May;271(5):2758-2767. doi: 10.1007/s00415-024-12209-3. Epub 2024 Feb 24. J Neurol. 2024. PMID: 38400914 Free PMC article. Clinical Trial.
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

