Distinct Cortical-Thalamic-Striatal Circuits through the Parafascicular Nucleus
- PMID: 30905392
- PMCID: PMC7164542
- DOI: 10.1016/j.neuron.2019.02.035
Distinct Cortical-Thalamic-Striatal Circuits through the Parafascicular Nucleus
Abstract
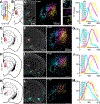
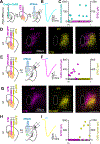
The thalamic parafascicular nucleus (PF), an excitatory input to the basal ganglia, is targeted with deep-brain stimulation to alleviate a range of neuropsychiatric symptoms. Furthermore, PF lesions disrupt the execution of correct motor actions in uncertain environments. Nevertheless, the circuitry of the PF and its contribution to action selection are poorly understood. We find that, in mice, PF has the highest density of striatum-projecting neurons among all sub-cortical structures. This projection arises from transcriptionally and physiologically distinct classes of PF neurons that are also reciprocally connected with functionally distinct cortical regions, differentially innervate striatal neurons, and are not synaptically connected in PF. Thus, mouse PF contains heterogeneous neurons that are organized into parallel and independent associative, limbic, and somatosensory circuits. Furthermore, these subcircuits share motifs of cortical-PF-cortical and cortical-PF-striatum organization that allow each PF subregion, via its precise connectivity with cortex, to coordinate diverse inputs to striatum.
Keywords: STPT; action selection; basal ganglia; corticothalamic loops; electrophysiology; parafascicular nucleus; single-cell transcriptional analysis; thalamus.
Copyright © 2019 Elsevier Inc. All rights reserved.
Figures






References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

